Hyperglycaemia-induced resistance to Docetaxel is negated by metformin: a role for IGFBP-2
- 1IGFs & Metabolic Endocrinology Group, School of Clinical Sciences, Learning & Research Building, Southmead Hospital, Bristol, UK
- 2Department of Urology, Southmead Hospital, Bristol, UK
- 3Department of Clinical Oncology, Bristol Haematology and Oncology Centre, University Hospitals Bristol, Bristol, UK
- Correspondence should be addressed to K Biernacka; Email: mdxkz{at}bristol.ac.uk
-
Figure 1
Dose response to metformin (0–10 mM) in PCa cell lines. Graphs show changes in % cell death in response to metformin. (A) DU145 cells were exposed to metformin in different glucose concentrations (5–25 mM). Cells were plated in six-well dishes (0.2 × 106 cells/well or 0.3 × 106 for LNCaP cells) in 5 mM glucose GM. The next day, media was switched to SFM containing different glucose concentrations (5 mM or 25 mM) for another 24 h. Cells were then dosed with metformin for a further 24 h. Dead cells were counted using trypan blue cell staining. (B) LNCaP, (C) PC3 and (D) VCaP cells were set up as described in Fig. 1A and levels of cell death were assessed using trypan blue staining. Graphs show mean of three experiments each repeated in triplicate.
-
Figure 2
Changes in prostate cancer cell morphology treated with metformin (2.5–7.5 mM). (A) DU145 cells were plated in T-25 flasks (0.3 × 106 cells/flask) in 5 mM glucose GM. The next day, media was switched to SFM containing different glucose concentrations (5 mM or 25 mM) for additional 24 h. Cells were then dosed with metformin for a further 24 h. Metformin induced a dose-dependent reduction in cell number and increased cell detachment in 5 mM but not in 25 mM glucose conditions. (B) LNCaP cells were set up as described in Fig. 2A and metformin induced a dose-dependent reduction in cell number in 5 mM but not in 25 mM glucose conditions. (C) VCaP cells were set up as described in Fig. 2A and metformin treatment induced some reduction in cell number in 5 mM but not in 25 mM glucose conditions. (D) PC3 cells were set up as described in Fig. 2A. Metformin did not alter cell number in either 5 mM or in 25 mM glucose conditions. All images were acquired under 10× magnification.
-
Figure 3
Changes in % cell death in response to single or combination treatment with Docetaxel (35–60 nM) and metformin (5 mM) in prostate cancer cell lines. (A) DU145 cells were set up as described in Fig. 1A and dosed with 35 nM Docetaxel, 5 mM metformin or with both for a further 24 h. (B) LNCaP cells were set up as described in Fig. 1A and dosed with 60 nM Docetaxel, 5 mM metformin or combined treatment for a further 24 h. (C) PC3 cells were set up as described in Fig. 1A and dosed with 45 nM Docetaxel, 5 mM metformin or both for a further 24 h. (D) VCaP cells were set up as described in Fig. 1A and treated with 45 nM Docetaxel, 5 mM metformin or both for another 24 h. For all figures, levels of % cell death were assessed using trypan blue cell counting. All graphs show mean of three experiments each repeated in triplicate.
-
Figure 4
(A) DU145, PC3, LNCaP and VCaP PCa cell lines were screened for abundance of LKB1 protein. Cells were grown in growth media and whole-cell lysates were prepared and subjected to Western blotting. (B) Changes in p-AMPK levels after metformin (2.5–7.5 mM) treatment. DU145 cells were seeded in 5 mM glucose for 24 h, which was replaced with either 5 mM or 25 mM SFM for a further 24 h. Cells were then treated with metformin (2.5–7.5 mM) for 24 h and whole-cell lysates were prepared and subjected to Western blotting. 50 µg of protein were loaded onto a 10% gel. (C) Shows levels of p-AMPK in PC3 cells exposed to metformin (2.5–7.5 mM) treatment. Cells were set up as described in Fig. 4B. (D) Shows levels of P-AMPK in LNCaP cells exposed to metformin (2.5 and 5 mM) treatment. Cells were set up as described in Fig. 4B. (E) Shows levels of p-AMPK in VCaP cells exposed to metformin (5 and 7.5 mM) treatment. Each blot is representative of experiments repeated three times, and the densitometry shows the changes of p-AMPK adjusted to loading control for all experiments (n = 3).
-
Figure 5
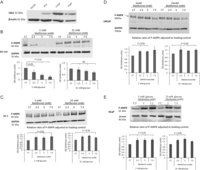
(A) Changes in mRNA levels of IGFB-2 in response to 5 mM metformin treatment in either 5 mM or 25 mM glucose conditions. DU145 and LNCaP cells were seeded at 0.8 × 106 cells/T-25 flasks and cultured as described in Fig. 1A, mRNA was extracted 24 h after dosing with metformin and Q-PCR was performed. Values of IGFBP-2 gene expression were normalised to the housekeeping gene (18S) (n = 3). (B) Western immunoblots show changes in the abundance of IGFBP-2 from DU145 or LNCaP cell lysates respectively, exposed for 24 h to (1–10 mM) metformin in either 5 mM or 25 mM glucose. Cells were seeded at 0.5 × 106 cells/T-25 flask and cultured as described in Fig. 1A and whole-cell lysates were collected and subjected to Western blot technique. Each blot is representative of experiments repeated three times, and the densitometry shows the mean changes (n = 3). (C) Western immunoblot shows changes in the abundance of IGFBP-2 from DU145 cell supernatants exposed for 24 h to (1–10 mM) metformin in either 5 mM or 25 mM glucose. Cells were set up as described in Fig. 1A, and conditioned media was collected and subjected to Western blot technique. Each blot is representative of experiments repeated three times and densitometry shows the mean changes at a representative dose of metformin (n = 3). (D) Western immunoblot shows changes in the abundance of IGFBP-2 in PC3 and VCaP cells. (E) Changes in mRNA levels of IGFBP-2 in response to 5 mM metformin treatment in either 5 mM or 25 mM glucose conditions. VCaP cells were seeded at 0.8 × 106 cells/T-25 flasks and cultured as described in Fig. 1A, mRNA was extracted 24 h after dosing with metformin and Q-PCR was performed. Values of IGFBP-2 gene expression were normalised to the housekeeping gene (18S) (n = 3). (F) Western immunoblots show changes in the abundance of IGFBP-2 from VCaP cell lysates respectively, exposed for 24 h to (5–7.5 mM) metformin in either 5 mM or 25 mM glucose. (G) Western immunoblot shows changes in the abundance of IGFBP-2 from VCAP cell supernatants exposed for 24 h to (2.5–7.5 mM) metformin in either 5 mM or 25 mM glucose.
-
Figure 6
Changes in % cell death in response to treatment of metformin and/or IGFBP-2. DU145 cells seeded in six-well plates at 0.2 × 106 cell/well with 5 mM glucose for 24 h. Cells were then transferred to 5 mM SFM and were treated for 24 h with 250 mM IGFBP-2 and/or 2.5–7.5 mM metformin. Cell death was assessed using trypan blue cell counting. Graph shows the mean of four experiments each repeated in triplicate.
-
Figure 7
Changes in IGFBP-2 gene expression in response to different treatments in either 5 mM or 25 mM glucose conditions. (A) LNCaP cells were set up as described in Fig. 5A and pre-treated with an AMPK inhibitor – Compound C (2 µM) for 1 h before dosing with 5 mM metformin for another 24 h. The mRNA was extracted and Q-PCR was performed. Values of IGFBP-2 gene expression were normalised to the housekeeping gene (18S) (n = 3). Western blot insert shows successful inhibition of metformin-induced AMPK phosphorylation by Compound C. (B) LNCaP cells were transfected with 75 nM siRNA for α1 and α2 subunits of AMPK or 25 mM non-silencing control, whereas seeding in six-well plates (0.3 × 106 cells/well) with 5 mM glucose and cultured as described in Fig. 1A. After 24 h treatment with 5 mM metformin, mRNA was extracted and Q-PCR was performed. Values of IGFBP-2 gene expression were normalised to the house-keeping gene (18S) (n = 3). (C) Changes in % cell death of LNCaP cells silenced with 75 nM siRNA for AMPK or 25 nM non-silencing control, whereas seeding in six-well plates (0.3 × 106 cells/well) with 5 mM glucose and cultured as described in Fig. 1A. After 24 h treatment with 60 nM Docetaxel for further 24 h, levels of cell death were assessed using trypan blue cell counting (n = 3). Western blot insert shows effective silencing of AMPK. (D) Changes in % cell death of LNCaP cells set up as described in Fig. 6C but silenced with 30 nM IGFBP-2 siRNA or 25 nM non-silencing control and treated with 5 mM metformin or/and with 60 nM Docetaxel for 24 h. Cell death was assessed by counting using trypan blue (n = 3). Western blot insert shows effective silencing of IGFBP-2.
- © 2017 The authors