ErbB-2 nuclear function in breast cancer growth, metastasis and resistance to therapy
-
Figure 1
ErbB-2 structure. Regions that are relevant to ErbB-2 function are indicated schematically. The extracellular portion is composed by 4 domains (I–IV). ErbB-2 contains a transmembrane domain (TMD) and a juxtamembrane domain (YMD), which encloses the nuclear localization sequence (NLS, purple). The intracellular domain includes the kinase domain (blue) and a proline-rich transactivation domain near the carboxy-terminus (pink). The major phosphorylation sites are indicated (yellow circles).
-
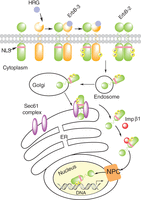
Figure 2
Proposed model of ligand (HRG)-induced and constitutive ErbB-2 nuclear transport from the cell surface to the nucleus. In this model, the Sec61 translocon, part of the ERAD pathway, associates with ErbB-2 at the endoplasmic reticulum (ER) and extracts full-length ErbB-2 from the membrane bilayer allowing its interaction with importin β1 (Imp β1). The ErbB-2/importin β1 complex then interacts with the nuclear pore protein Nup358, a member of the nuclear pore complex (NPC), which results in ErbB-2 nuclear translocation. NLS, nuclear localization sequence.
-
Figure 3
Stat3/ErbB-2/PR transcriptional complex induced by progestins in breast cancer. PR activated by progestins induces the phosphorylation of ErbB-2 at Tyr 1222 and Tyr 877 (1). ErbB-2 activated by a nongenomic PR action stimulates Stat3 phosphorylation (2) (Beguelin et al. 2010). ErbB-2 (3) and Stat3 (4) then translocate to the nucleus where they assemble a transcriptional complex at the Stat3 response element (GAS sites) of the cyclin D1 promoter, in which ErbB-2 acts as a coactivator of Stat3. PR is also loaded on the Stat3/ErbB-2 complex (5).
-
Figure 4
Nuclear ErbB-2’s role in the response to trastuzumab (TZ). BC cells sensitive to TZ show higher levels of MErbB-2 than those of NErbB-2. Their proliferation is stimulated by ErbB-2 signaling (e.g. PI3K/AKT) and nuclear (e.g. Stat3/ErbB-2/ErbB-3 transcriptional complex at cyclin D1 promoter) actions, both hierarchically equal. Inhibition of signaling with TZ or of nuclear action with hErbB-2ΔNLS inhibits proliferation. TZ-resistant cells show high levels of NErbB-2, which may be higher than those of MErbB-2. Nuclear ErbB-2 effect on proliferation is stronger than that of MErbB-2. Blockade of nuclear ErbB-2 effect with hErbB-2ΔNLS prevents BC growth in spite of the activation of signaling cascades.
- © 2016 Society for Endocrinology