Hormonal contraception and risk of endometrial cancer: a systematic review
- Department of Endocrinology and Menopause, Centre for Women's Health BW, University Women's Hospital, Calwer Strasse 7, D-72076 Tuebingen, Germany
- (Correspondence should be addressed to A O Mueck; Email: alfred.mueck{at}med.uni-tuebingen.de)
Abstract
More than 15 case–control studies and at least four large cohort studies demonstrated a decrease in the risk of endometrial cancer of about 50% for ever use of combined oral contraceptives (COCs). In most of these studies, this protective effect persisted for more than 10–15–20 years after cessation of the COC. An increasing protective effect with longer duration of COC use has been found in most studies. The beneficial effect was independent of the composition of COC, i.e. dosage and type of progestogen, combined with ethinyl estradiol 30–50 μg/day. COCs with higher progestogen potency seem to be somewhat more effective. Nonhormonal uterine devices have also been found to be strongly protective; however, data on oral or injectable progestogen-only preparations (POPs) including the levonorgestrel-releasing intrauterine system (LNG-IUS) are still rare, but also suggest similar protective action. COCs, POPs, as well as LNG-IUS can effectively reduce endometrial hyperplasia but should only be used in exceptional cases in patients with or after endometrial cancer. In contrast to nonhormonal IUS, systemic side effects cannot be excluded with LNG-IUS, but they are certainly rare, as the main effect has decreased the endometrial estrogen response because of the high endometrial tissue levels of LNG.
Introduction
Two different clinicopathological subtypes of endometrial cancer are recognized: estrogen-related type 1 (endometroid), comprising 70–80% of newly diagnosed cancer, and nonestrogen-related type 2 (nonendometroid such as papillary serous and clear cell).
The biological basis of type 1 is that estrogen stimulates endometrial cell division, whereas progestogens block this effect. As a result of progestogen action, cell proliferation ceases, despite continuous exposure to estrogen levels (as in the luteal phase). Progestogens protect from estrogen-induced hyperplasia and changes in proliferative status. They induce glandular epithelial secretory activity and decidual transformation of stromal fibroblasts; these terminal-differentiated cells can no longer proliferate and are shed in withdrawal bleeding (if implantation does not occur), with important differences dependent on the pharmacology of the progestogens used (type, dosage, pharmacokinetics, etc).
However, many more changes in histological features occur during hormonal contraception treatment, such as different proliferatory, secretory, and atrophic (like) patterns, changes in gland-to-stroma ratio, stromal factors (e.g. very potent growth factors), architectural structures (e.g. cribiform and/or papillary patterns), glandular cellularity, cytoplasmic changes, mitotic activity, (tumor-)angiogenesis, and increase or decrease in cytologic atypia; the latter are powerful markers and predictors for progestogenic potency.
Most, if not all, of these effects, which are still a topic of important ongoing research, suggest that hormonal contraceptives can reduce the risk of endometrial cancer. To demonstrate a causal relationship in clinical studies would require randomized placebo-controlled interventional studies, which are not ethical in the field of contraception. Thus, the aim of this systematic review was to search for observational studies with a focus on case–control and cohort studies and to summarize the results, especially those of the most important (large) studies. To our knowledge, the last systematic review on this topic was published more than 15 years ago.
Although observational studies can only evaluate associations, not causality between cancer risk and hormonal use in this case, it is very important for daily practice to know if we have to expect an increase or decrease in the risk, and how great the evidence is, which points in one or the other direction.
Method of systematic review
The literature review included a search in MEDLINE from the start of this database in January 1969. This was the prime source for this report. In addition, we searched the PubMed and EMBASE databases for studies after 1980. Key words were (‘contraceptives’ or ‘exogenous hormones’) and (‘endometrial cancer’ or ‘neoplasm’) and (‘case–control study’ or ‘cohort study’). The search was primarily limited to English language articles. To be considered for inclusion, publications had to be original articles.
We included all cohort and case–control studies up to December 2009. We retrieved and assessed potentially relevant articles, and checked the reference lists of all papers of interest to identify additional relevant publications. At least, two of the authors selected and extracted the studies followed by double checking both literature searches and data extraction.
Studies were only included if they considered information on contraceptives separately from hormone replacement therapy (HRT) or other hormonal therapies. We did not consider abstracts and case reports regarding studies with combined oral contraceptives (COCs), but due to the lack of data, we also searched for case reports about the risk with progestogen-only preparations (POPs) and levonorgestrel-releasing intrauterine system (LNG-IUS), which were published in well-known journals. Accordingly, because of the lack of data, we searched for progestogen-only contraception (especially including LNG-IUS) with the keyword ‘endometrial hyperplasia’.
The present review is of qualitative and empirical nature, and no statistical analysis was used to compare the various studies. The prime objective was to evaluate whether there is a strong association between increased or decreased risk of endometrial cancer and contraceptive use. We did not check for adjustments in the various studies, although it is well known that factors such as age, family history, parity, smoking, genetics, and body mass index (BMI) have been shown as basic risk factors for endometrial cancer. Most studies using hormonal contraceptives have been adjusted for the most important factors that increase endometrial cancer risk such as age, BMI, family history, and smoking. However, the modulating effect of these factors on the risk profile during use of hormonal contraception has only been investigated in a few studies with small subgroup numbers. All in all, it seems that these factors have only a minor influence, if at all, on the protective effect of hormonal contraceptives.
Early case–control and cohort studies
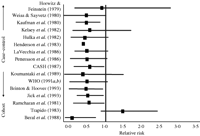
The first relevant systematic review using the criteria of the US Preventive Services Task Force and evaluating the association between COCs and endometrial cancer was published in 1995 (Grimes & Economy 1995), assessing 13 case–control studies (Horwitz & Feinstein 1979, Kaufman et al. 1980, Weiss & Sayvetz 1980, Hulka et al. 1982, Kelsey et al. 1982, Henderson et al. 1983, LaVecchia et al. 1986, Pettersson et al. 1986, CASH 1987, Koumantaki et al. 1989, WHO Collaborative Study 1991a,b, Brinton & Hoover 1993, Jick et al. 1993, Maxwell et al. 2006) and 3 cohort studies (Ramcharan et al. 1981, Trapido 1983, Beral et al. 1988; Fig. 1).
Risk of endometrial cancer associated with ever use of combined oral contraceptives modified from American Journal of Obstetrics and Gynecology, Grimes & Economy, Primary prevention of gynecologic cancers, Copyright (1995), with permission from Elsevier.
The first report, published in 1979, was a case–control study (n=268/268, Yale Registry, USA) and has failed to support an association between COC use and endometrial cancer (odds ratio (OR) 0.95). However, all the other early case–control studies found a protective effect ranging from OR 0.1 to 0.6, with most clustering around 0.5. In several reports, the protection was significant (Kaufman et al. 1980, CASH 1987, Brinton & Hoover 1993, Jick et al. 1993) including the largest case–control study and the Cancer and Steroid Hormone Study of the Centers for Disease Control (CASH) (CASH 1987).
Only one study, a cohort study in Eastern Massachusetts, found a modest, nonsignificant increase in risk (Trapido 1983), but included high-dose sequential preparations (100 μg ethinyl estradiol (EE)) combined with low-dose, short sequential progestin, which has been off the market for over 20 years. Two of the three cohort studies reported a significant protective effect. These included the Walnut Creek Contraceptive Drug Study from California (Ramcharan et al. 1981) and the Study from the Royal College of General Practitioners (Beral et al. 1988). This UK study is the most important cohort study, evaluating the 47 000 women 1988 report, that found an 80% reduction in risk (relative risk (RR) 0.2; 95% CI 0.0–0.7).
Important studies on the risk of using COC
Only a few more relevant studies have been published up to now, which also investigated the follow-up of earlier large studies and risk factors, which could possibly modulate the effect of hormonal contraceptive use. Table 1 summarizes the most important studies in chronological sequence of publications.
Risk of endometrial cancer during oral contraception (relevant studies listed in chronological sequence)
The most important study using incident cohort data in large patient samples has been the Royal College Study (Beral et al. 1988), and the recently published data followed 45 950 women for a mean of 24 years (Hannaford et al. 2007). The data came from 6-monthly reports from the women's general practitioners until 1996, and from linkage of 35 050 women who were still in the study in the mid-1970s to National Health Service central registries. The main dataset contained about 339 000 woman-years of observation for never users and 744 000 woman-years for ever users. Most of the pills were COCs, only 3% were progestin-only pills (POP).
Compared with never users, ever users had statistically significant lower rates of cancers of the uterine body, calculated in the main dataset with RR 0.58 (95% CI 0.42–0.79), standardized rate per 100 000 woman-years 11.30/19.53 (adjusted for age, parity, smoking, and social status). The risk of duration of OC use was also assessed (general practitioner dataset). Even with small numbers, the trend for longer use was statistically significant. With regard to recent use, only <5 years after stopping using oral contraception reached significance. As only 566 women exclusively used COC containing more than 50 μg EE, this study cannot answer the question of how much the risk is dependent on the hormonal potencies of the COC.
This question was specifically investigated in the WHO Collaborative Study (1991a,b), which classified COC according to the dosage of EE and potency/dosage of progestin. High-dose EE/low progestin did not alter the risk (OR 1.10; 95% CI 0.13–9.96), in contrast (not significant) to low-dose EE/high progestin (OR 0.0; 95% CI 0–1.08) and low EE/low progestin (OR 0.59; 95% CI 0.26–1.30). High-dose versus low-dose progestin significantly decreased the risk (OR 0.21; 95% CI 0.05–0.84).
The multicenter, population-based case–control study with the longest duration up to now is the still ongoing CASH study, with enrollment between 1980 and 1982 at eight US regional cancer registries participating in the Surveillance Epidemiology Program of the National Cancer Institute. The first larger evaluation in 1987 (CASH 1987) included 433 cases and 3191 controls, limited to women aged 20–54 years. Women who had used OCs for at least 1 year had an age-adjusted risk of 0.6 (95% CI 0.3–0.9). This protective effect persisted for at least 15 years after cessation of COC use. The protective effect was independent of the type of histology.
The latest evaluation of CASH (Maxwell et al. 2006) also focused on the hormonal potencies, including 434 endometrial cancer cases and 2557 controls. Compared with nonusers, high-progestin COC as well as low-progestin COC significantly decreased the risk (OR 0.21; 95% CI 0.10–0.43 and OR 0.39; 95% CI 0.25–0.60 respectively), but only high-progestin COCs were protective in women with a BMI >22 (OR 0.31; 95% CI 0.11–0.92).
Likewise, in a large population-based Swedish case–control study (n=709/3368) (Weiderpass et al. 1999), high-, medium-, and low-dose progestin COC reduced the risk, although it was only significant with high and medium dosages (adjusted OR 0.7; 95% CI 0.5–0.9). This protective effect was similar for all degrees of tumor differentiation and invasiveness. As only postmenopausal women aged 50–74 years were investigated, subsequent use of HRT was also assessed, and was not found to modify the protective effect of the COC used at a younger age. The reduction in risk was noticeable after 3 years of use (OR 0.5; 95% CI 0.3–0.7), and increased with duration of intake, reaching 80% lower risk after 10 years of use (OR 0.2; 95% CI 0.1–0.4), and as in the CASH study, the protective effect remained for at least 15–20 years after cessation of COC.
Almost similar results have been found in a German population-based case–control study (n=485/1570; Heinemann et al. 2003) with the reduction in risk comparable for all COCs used (adjusted OR 0.36; 95% CI 0.28–0.45, ever versus never use) comparable to low-dose COC (OR 0.30; 95% CI 0.12–0.74), and a protective effect starting within 5 years (OR 0.63; 95% CI 0.47–0.86), increasing with duration of use, reaching 75% lower risk after 10 years (OR 0.25; 95% CI 0.18–0.34), and persisting for more then 10 years after stopping pill use.
Similar trends were also observed in a large recent Chinese case–control study (Tao et al. 2006; n=1.204/1.212). The risk for ever users of COC was decreased (OR 0.75; 95% CI 0.60–0.93), the protective effect increased with duration of use (5 years or more: OR 0.50; 95% CI 0.30–0.85) and remained for 25 years after cessation of use (OR 0.57; 95% CI 0.42–0.78). Similar results have been found in further case–control studies, which are listed in Table 1 (WHO 1988, Levi et al. 1991, Stanford et al. 1993).
The 2006 update on the Oxford family planning association cohort study evaluating 17 032 women has been published recently (77 cases), with a more than 50% reduction in ever users (RR for 97+ months 0.1 (95% CI 0.0–0.4)) and a protective effect lasting for more than 20 years after cessation of OCs. In this analysis (Vessey & Painter 2006), the data for the cancers of the cervix, uterine body, and ovary were also combined, resulting in an age-adjusted RR 0.7 (95% CI 0.5–0.8).
Time dependency of risk reduction
Increasing protective effects with duration of COC use have been found in most studies that have investigated this issue (Table 2). A systematic meta-analysis (Schlesselman 1997) including ten case–control studies (Kaufman et al. 1980, Weiss & Sayvetz 1980, Hulka et al. 1982, Kelsey et al. 1982, Henderson et al. 1983, LaVecchia et al. 1986, Pettersson et al. 1986, CASH 1987, Levi et al. 1991, Stanford et al. 1993) and the cohort study of the Royal College of General Practitioners (Beral et al. 1988) calculated a significant reduction of risk with RR 0.44, 0.33, and 0.28 after 4, 8, and 12 years of COC use respectively based on 33 time-dependent estimates of RR, adjusted for age, adiposity, parity, and use of estrogen replacement therapy. The trend of decreasing risk with increasing duration of use of COC was highly significant (P<0.0001, one sided).
Data on relative risk (RR) of invasive endometrial cancer in relation to duration of use of combined oral contraceptives
In this meta-analysis, the adjusted RRs by recency of use of COC were also calculated, based on 19 estimates of RRs. After stopping COC, the decrease in risk persisted for 20 years after discontinuation, and the trend for decrease in risk reduction was significant (P=0.011, one sided), but still remained about 50% (RR 0.33, 0.41, and 0.51 for 5, 10, and 20 years after pill cessation respectively). Of interest, seems the fact that the residual protective effect from prior COC use continues throughout the menopause, at a time when the risk of endometrial cancer is greatest.
Risk using oral and injectable progestin-only preparations
All the studies listed in Fig. 1 investigated the effect of COC; progestin-only preparation (POP) users were only separately tabulated in the CASH and WHO studies, with very small numbers (WHO 1991a,b, Maxwell et al. 2006). In CASH, only one case and six controls had used POPs exclusively, resulting in a crude OR of 0.6 (95% CI 0.1–5.0). The WHO Collaborative Study of Neoplasia and Steroid Contraception, a hospital-based case–control study comparing 220 cases from 7 countries with 1537 age-matched controls, found no cases and only 2 controls who had exclusively used oral POPs.
Newer studies also included only small patient samples. As only 3% were POP users in the large cohort study from the Royal College, risks have not been calculated (Hannaford et al. 2007). In a large Swedish case–control study (Weiderpass et al. 1999) from 707 cases/3368 controls, only 7/61 used the mini pill (OR 0.4; 95% CI 0.2–1.4) and 0/14 depot-medroxyprogesterone acetate (DMPA).
Up to now, we have very little knowledge about the protective effect of using POPs. Therefore, inferences about POPs must be made from knowledge about COCs, other risk factors, and biological mechanisms.
Risk using nonhormonal and hormonal intrauterine devices
A meta-analysis has been published recently (Beining et al. 2008), based on a comprehensive search of literature published up to 2007, to examine the association on ever use of different nonhormonal intrauterine devices (IUDs) and endometrial cancer. By pooling ten studies, a strong protective effect from IUDs was observed (pooled adjusted OR 0.54; 95% CI 0.47–0.63). With the exception of one small Chinese study (Shu et al. 1991; OR 1.10; 95% CI 0.50–2.50), the protection was seen in all the studies (seven significant), increased with the duration of use, and was still to be observed 5 years after cessation of use (OR 0.91; 95% CI 0.86–0.95). Only three studies reported on specific types of the nonhormonal IUD used (copper, Lippes loop, Dalkon Shield, Majzlin spring, and stainless steel); thus, the data for types were too sparse to pool.
The use of the LNG-IUS to protect from endometrial cancer is one of the most important issues regarding cancer risk and contraception (Hubacher & Grimes 2002) as well as HRT (Riphagen 2000). However, studies on primary risk reduction in general populations are still lacking, although in 2008, at least three studies and two case reports for effective treatment of endometrial hyperplasia (including even atypical or complex histology) were published (Haimovich et al. 2008, Oerbo et al. 2008, Qi et al. 2008, Varma et al. 2008), in addition to a series of earlier reports (Vereide et al. 2003, 2005, Giannopoulos et al. 2004, Wildemeersch et al. 2007).
To our knowledge, only very few case reports, but no special study, raised doubts about the effectiveness of LNG-IUS for treatment of endometrial hyperplasia. However, even endometrial protection against tamoxifen-stimulated endometrial changes by using LNG-IUS was demonstrated in several studies (Gardner et al. 2000, Chan et al. 2007, Kesim et al. 2008). All in all, it can be concluded that LNG-IUS is a promising alternative to hysterectomy for the treatment of endometrial hyperplasia, although data are too sparse to recommend treatment of early endometrial cancer, and case reports are controversial (Parazzini et al. 1994, Sturgeon et al. 1997, Varila et al. 2001, Giannopoulos et al. 2004, Dhar et al. 2005, Kresowik et al. 2008).
Mechanisms using nonhormonal and hormonal IUDs
It can be expected that LNG-IUS used for contraception protects primarily from endometrial cancer as do nonhormonal IUDs, although the mechanism differs between these IUSs (Aikat & Chadda 1980, Nilsson et al. 1982, Johannisson 1987, Castellsague et al. 1993, Raudaskoski et al. 1998, Phillips et al. 2003, Vereide et al. 2005, 2006). Regarding the possible mechanisms of protection, there are important differences between nonhormonal IUDs and the (until now only available) hormonal LNG-IUS: although local intrauterine changes are suggested to be the main effect for contraception with both systems within a few days after insertion, only local endometrial effects without relevant systemic side effects (e.g. no relevant copper absorption) are associated with nonhormonal IUDs (Beining et al. 2008). The mechanisms of nonhormonal IUDs are to develop local chronic inflammation leading to inhibition of nidation, and perhaps in addition also releasing mediators that have a toxic impact on sperm motility and sperm metabolism, although until now the detailed understanding has been a matter of intensive research with some questions remaining open.
In contrast, systemic effects cannot be excluded when using the LNG-IUS. Mean plasma levels of levonorgestrel can be assessed to be in the range of about 150–200 pg/ml (0.4–0.6 nmol/l), whereas endometrial tissue levels more than tenfold higher are obtained (Riphagen 2000). Thus, side effects similar to those seen with orally or parenterally administered progestin-only preparations have been observed in the studies, but are very rare. For practical use, it seems important that the same contraindications are labeled for the use of LNG-IUS as with other progestin-only preparations, and have to be considered when attaining patients' informed consent.
However, the main effect of LNG-IUS is to decrease the local endometrial response to estrogen, as with the use of systemically administered progestins when added to estrogen therapy (Pike & Spicer 2000, Riphagen 2000). During progestogen action, cell proliferation ceases despite continuous exposure to estrogen levels (as in the luteal phase). Progestogens protect from estrogen-induced hyperplasia and changes in proliferative status. They induce glandular epithelial secretory activity and decidual transformation of stromal fibroblasts; these terminally differentiated cells can no longer proliferate and are shed in withdrawal bleeding (if implantation does not occur), with strong differences dependent on the pharmacology of progestogens used (type, dosage, pharmacokinetics, etc; Pike & Spicer 2000). This is suggested to be the main mechanism regarding endometrial effects of all progestin-only preparations. With longer use, there are atrophic changes combined with amenorrhea. In most cases, this occurs within 6–12 months of LNG-IUS insertion. However, if this can lead to primary protection from endometrial cancer, then clinical end point studies into LNG-IUS and endometrial protection are urgently needed.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review reported.
Funding
This review did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
- © 2010 Society for Endocrinology