Very low-density lipoprotein (VLDL)-induced signals mediating aldosterone production
- 1Department of Physiology, Medical College of Georgia at Augusta University (formerly Georgia Regents University), Augusta, Georgia, USA
- 2Departments of Molecular & Integrative Physiology and Internal Medicine, University of Michigan, Ann Arbor, Michigan, USA
- 3Charlie Norwood VA Medical Center, One Freedom Way, Augusta, Georgia, USA
- Correspondence should be addressed to W B Bollag; Email: wbollag{at}augusta.edu
-
Figure 1
Steroidogenesis in the adrenal cortex. Shown are the pathways by which the adrenal cortex synthesizes aldosterone in the zona glomerulosa, cortisol in the zona fasciculata and dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEA-S) in the zona reticularis. All steroid hormones are synthesized from cholesterol, which is transported by the protein steroidogenic acute regulatory (StAR) protein from the outer to the inner mitochondrial membrane, where the initial step of side-chain cleavage is catalyzed by CYP11A1 to yield pregnenolone. CYP17 is an enzyme with two activities, hydroxylation and lyase, which allows the synthesis of cortisol and DHEA. CYP11B2 is expressed in the zona glomerulosa where the protein encoded by this gene, aldosterone synthase, is required for aldosterone production, catalyzing the last three reactions in aldosterone biosynthesis, 11-hydroxylation followed by 18-hydroxylation and subsequent oxidation to yield an aldehyde. CYP11B1, on the other hand, produces cortisol in the zona fasciculata.
-
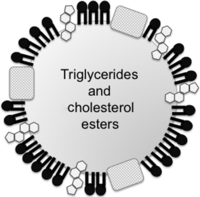
Figure 2
Schematic structure of lipoproteins. Lipoproteins are composed of cholesterol, phospholipids and apolipoproteins surrounding a hydrophobic core of triglycerides and cholesteryl esters. VLDL has a greater diameter (with a very low density) and contains a large amount of triglyceride and small amounts of cholesteryl ester in its core. VLDL is metabolized to IDL and then to LDL, which has a smaller diameter (with a greater density than VLDL or IDL) and a large proportion of cholesteryl esters with small amounts of triglycerides (reviewed in Feingold & Grunfeld 2015).
-
Figure 3
Traditional regulators of aldosterone production and their signaling pathways. The traditional aldosterone secretagogues are angiotensin II (AngII), elevated extracellular potassium (K+) levels and adrenocorticotrophic hormone (ACTH), which function through different signal transduction pathways. (A) AngII binds to angiotensin II type 1 receptors (AT1R) to activate phosphoinositide-specific phospholipase C (PLC), which hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to generate inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 binds to IP3 receptors (IP3R) on the endoplasmic reticulum (ER) to release ER calcium (Ca2+) ions and increase cytosolic Ca2+ levels. The increase in intracellular Ca2+ concentration activates Ca2+/calmodulin-dependent protein kinases (CaMK) that can phosphorylate and activate various members of the cAMP response element-binding protein (CREB)/ activating transcription factor (ATF) family of transcription factors (represented as CREB). This family, as well as Nurr1, the levels of which are also elevated, can induce the expression of genes encoding steroidogenic proteins, such as steroidogenic acute regulatory protein (StAR) and CYP11B2 (coding for aldosterone synthase). AngII also increases Ca2+ influx through voltage-dependent Ca2+ channels and store-operated Ca2+ channels. The other second messenger produced by PLC activity, DAG, activates the protein kinase C (PKC) family of isoenzymes, some of which phosphorylate and activate protein kinase D (PKD); PKD is also known to phosphorylate and activate members of the CREB family of transcription factors. PKC can also activate extracellular signal-regulated kinase-1/2 (ERK), which is able to phosphorylate and activate cholesterol ester hydrolase, to release cholesterol from its storage as cholesteryl ester in lipid droplets, and likely also StAR. DAG can also be generated from the phosphatidic acid (PA) produced by phospholipase D (PLD), which is also activated by AngII and underlies steroidogenesis, although PA can itself serve as a second messenger to mediate the activation of various enzymes (reviewed in Bollag 2016). In addition, AngII working through the AT1R activates Src family kinases (SFK), which can also stimulate PKD activity and appear to underlie aldosterone production. Finally, AngII can also activate JAK2 (not shown). (B) An elevated extracellular K+ level uses many of the same signal transduction pathways as AngII. In this case, an increased K+ concentration depolarizes the glomerulosa cell to open voltage-dependent Ca2+ channels, increase intracellular Ca2+ levels and activate CaMK, with its downstream targets. There is evidence that K+, like AngII, may also induce phosphoinositide hydrolysis, through an unknown mechanism, although controversy remains concerning this point. Elevated K+ also seems to activate PKC and PLD (Betancourt-Calle et al. 2001), which likely play similar roles as in AngII’s effects. Another signal that may or may not be used by elevated K+ levels to mediate steroidogenesis is the adenylate cyclase (AC)/cAMP/cAMP-dependent protein kinase (also known as protein kinase A or PKA) pathway. PKA is also known to phosphorylate and activate CREB family transcription factors as well as StAR. (C) ACTH stimulates aldosterone production by binding to its receptor, the melanocortin 2 receptor (MC2R), to activate AC that converts ATP to cAMP. cAMP then stimulates the activity of PKA, thereby activating CREB family transcription factors and StAR. ACTH also increases Ca2+ influx through an unclear mechanism, and the resulting increased Ca2+ cytosolic levels can activate CaMK, with its downstream effectors. These pathways have been recently reviewed in Bollag (2014).
-
Figure 4
The VLDL-activated pathway to aldosterone production in the zona glomerulosa. (A) Shown are the early effects of VLDL that promote acute aldosterone secretion. (1) Very low-density lipoprotein (VLDL) apparently binds to the scavenger receptor, class B, type 1 (SR-B1) and (2) activates, through an unknown mechanism, a phosphoinositide-specific phospholipase C (PI-PLC) that cleaves phosphatidylinositol 4,5-bisphosphate (PIP2) to yield inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). Various signaling cascades are then initiated simultaneously. For example, IP3 binds to IP3 receptors (IP3R) on the endoplasmic reticulum to release the stored calcium and increase cytosolic calcium levels (occurring within a few minutes of VLDL exposure); the increased cytosolic calcium levels activate calcium/calmodulin-dependent protein kinase (CaMK). At the same time, the other signal formed within minutes is DAG; DAG activates protein kinase C (PKC) isoforms to stimulate the activity of extracellular signal-regulated kinase (ERK), again within minutes of VLDL treatment. VLDL-stimulated ERK, like the angiotensin II (AngII)-activated enzyme, may, in turn, phosphorylate and activate cholesterol ester hydrolase (Cherradi et al. 2003), also known as hormone-sensitive lipase, to release cholesterol esters stored in lipid droplets. ERK also likely phosphorylates StAR, thereby enhancing its cholesterol transport activity (Poderoso et al. 2008). In addition, within minutes of VLDL exposure, activated PKC induces phosphorylation and activation of members of the activating transcription factor/cAMP response element binding protein family of transcription factors (ATF/CREB) to increase after a few hours the levels of steroidogenic acute regulatory protein (StAR). StAR allows transport of cholesterol into the inner mitochondrial membrane where it can be acted upon by the cholesterol side-chain cleavage complex (CYP11A1) located there to initiate steroidogenesis. (B) The initial (1) binding of VLDL to SRB1 and (2) activation of PI-PLC also activates phospholipase D (PLD) (within an hour, the earliest time tested) via an unknown mechanism. PLD catalyzes phosphatidylcholine hydrolysis to yield phosphatidic acid (PA), which can be dephosphorylated to DAG (by lipins) to sustain PKC activation and aldosterone production. PA may also have its own effector enzymes, such as Raf-1, a protein kinase upstream of ERK, as reviewed in Bollag (2014). PKC-elicited phosphorylation of ATF/CREB also induces the expression not only of StAR but also of aldosterone synthase, encoded by the gene CYP11B2, after 6–12 h. (By analogy to angiotensin II, CaMK also may induce the expression of members of this family (Felizola et al. 2014). CYP11B2 transcription is also promoted by the VLDL-induced increase in the mRNA and protein expression of the transcription factor Nurr1 (the protein levels of which are elevated after several hours of VLDL treatment). CYP11B2 in the mitochondria catalyzes the final steps in the production of aldosterone.
- © 2017 Society for Endocrinology