Getting to the heart of intracellular glucocorticoid regeneration: 11β-HSD1 in the myocardium
-
Figure 1
Adrenocorticosteroids, 11β-HSD1 and the heart. Hypothalamic-pituitary-adrenal (HPA) axis-derived glucocorticoids (cortisol in man, corticosterone in rats and mice) and the mineralocorticoid - aldosterone - compete for binding to cardiac glucocorticoid (GR) and mineralocorticoid receptors (MR). Glucocorticoids are also regenerated within the heart from circulating inert metabolites (cortisone in man and 11-dehydrocorticosterone (11-DHC) in rats and mice) by 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), when it is expressed alongside hexose-6-phosphate dehydrogenase (H6PDH). As the glucocorticoid concentration ([cort]) normally far exceeds that of aldosterone ([aldo]), GR and MR are usually occupied by glucocorticoids. 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) inactivates glucocorticoids, but unlike other MR target tissues, there is normally little or no dehydrogenase activity in the heart. Inactivation of glucocorticoids by 11β-HSD2 activity elsewhere in the body, largely in the kidney, generates the inert precursors that, on entering the circulation, become available for glucocorticoid regeneration and GR/MR activation in cells that express 11β-HSD1. In the healthy heart, 11β-HSD1 immunoreactivity (inset panel) is localised to vascular smooth muscle (VSM), fibroblasts (F) and also cardiomyocytes (CM) in a fixed section from mouse left ventricle. The 11β-HSD1 antibody is a sheep anti-mouse polyclonal generated in house (De Sousa Peixoto et al. 2008). Staining is notably absent in endothelial cells (EC) lining the vascular wall. A negative control of the same section generated using sheep IgG is shown in the inset panel. From S J McSweeney, PhD thesis, 2010 (McSweeney 2010). 11β-HSD1 expression can be increased by glucocorticoids, by pro-inflammatory Cytokines and in ageing in other tissues and this is also likely to be the case in the heart.
-
Figure 2
Hearts from adult mice with global 11β-HSD1 deficiency are hypomorphic and have shorter cardiomyocytes. Ultrasound analysis (A) reveals a significant reduction in left ventricular dimensions in 12-week-old Hsd11b1−/− (open bars) compared with control wild-type mice (filled bars). Reduction in heart size was confirmed when post-mortem heart weight was compared with body weight (B). LV dimensions (left ventricular end-diastolic area, solid red (Hsd11b1−/−) and black (control wild type), and left ventricular end-systolic area, hatched red (Hsd11b1−/−) and black (control wild type)) were not influenced by the loss of 11β-HSD1 from late gestation until 6 weeks of age (C). In adult hearts, there was no effect of 11β-HSD1 deficiency (red) on the distribution of cardiomyocyte cross-sectional area (D), but the length of individual cardiomyocytes (E) isolated from Hsd11b1−/− hearts was significantly less than that isolated from control wild-type hearts (black).*P < 0.05, ***P < 0.005, n = 5–8.
-
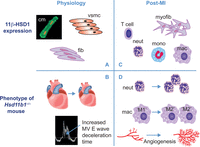
Figure 3
Distribution and actions of 11β-HSD1 in physiology and post-MI. In the healthy heart (panel A), 11β-HSD1 is expressed in cardiomyocytes (cm), vascular smooth muscle (VSMC) and fibroblasts (fib). Deletion of 11β-HSD1 (panel B) results in smaller adult hearts and mild diastolic dysfunction (identified by increased deceleration time on Doppler mitral valve (MV) ultrasound). After MI (panel C), 11β-HSD1 is additionally expressed in myofibroblasts (myofib) and recruited inflammatory cells (T cells, neutrophils (neut), monocyte (mono) and macrophages (mac)). 11β-HSD1 deficiency after MI (panel D) results in increased early neutrophil (neut) recruitment, increased acquisition of pro-repair ‘M2’ macrophage (mac) phenotype, relative to pro-inflammatory ‘M1’ macrophage phenotype and increased angiogenesis, leading to increased vessel density.
- © 2017 The authors