Bisphenol A and its analogues disrupt centrosome cycle and microtubule dynamics in prostate cancer
- 1Department of Environmental Health, University of Cincinnati Medical Center, Cincinnati, Ohio, USA
- 2Center for Environmental Genetics, University of Cincinnati Medical Center, Cincinnati, Ohio, USA
- 3Cincinnati Cancer Center, Cincinnati, Ohio, USA
- 4Cincinnati Veteran Affairs Hospital Medical Center, Cincinnati, Ohio, USA
- 5Center for Cancer Research, Hudson Institute of Medical Research, Clayton, Victoria, Australia
- 6Monash University, Clayton, Victoria, Australia
- Correspondence should be addressed to S-M Ho or P Tarapore; Email: shuk-mei.ho{at}uc.edu or pheruza.tarapore{at}uc.edu
-
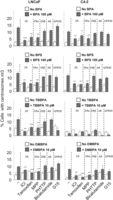
Figure 1
Low doses of BPA, BPS, TBBPA and DMBPA promote CA: LNCaP (A) and C4-2 (B) cell lines were treated with various doses of BPA, BPF, BPAF, BPS, TBBPA, DMBPA or TMBPA for 72 h in 10% CSS media. Cells were fixed and immunostained for centrosomes and DNA. Using fluorescence microscopy, cells with amplified centrosomes were scored and plotted for each dose of 0, 0.01, 0.1, 1, 10 and 100 nM. Significance was determined using the Student’s t-test (*P < 0.05). Bars, s.e. of the three independent experiments.
-
Figure 2
Representative staining showing cells with one, two and >2 centrosomes. LNCaP and C4-2 PCa cells were treated with (A) BPA, (B) BPS, (C) TBBPA and (D) DMBPA as indicated. Cells were fixed and immunostained with rabbit anti-γ-tubulin (red). DNA was stained using DAPI (blue). Arrows point to centrosomes (n = number of centrosomes). Pericentrin staining gave similar results. Scale bar, 20 μm. Area in square is magnified and offset to the main panel.
-
Figure 3
BPA promotes the initiation of centrosome duplication at an earlier time-point during G1 phase: LNCaP and C4-2 cells were serum starved in the presence of 0.5% CSS, media changed to 20% CSS ± BPA, BPS, TBBPA and DMBPA, and cells fixed at the time-points indicated in graph. At each time-point, cells were scored for centriole and centrosome numbers in LNCaP and C4-2 cell lines. (A) Scoring of number of cells with amplified centrosomes. (B) Scoring of number of cells with single centrosome. (C) Scoring of number of cells with duplicated centrioles. Around 5–10% of cells had separated centrioles at 0, 8 and 16-h time-points. (D) Representative staining showing cells with NPM on single centrosomes. Unduplicated (a–c, g–i) and duplicated (d–f, j–l) LNCaP (a–f) and C4-2 (g–l) cells were immunostained for gamma-tubulin (green, a, d, g, j), NPM (red, b, e, h, k) and DAPI (blue). DAPI images (blue) were merged with gamma-tubulin (green) and NPM (red). Arrows indicate the positions of the centrosome (n = number of centrosomes). Scale bar, 20 μm. (E) Cells were scored for the presence or absence of NPM on non-duplicated centrosomes at various time-points in LNCaP and C4-2 cell lines. (F) Relative expression as determined by qRT-PCR for p53, CDK4, CDK6, CDK2, p21Waf1, p27kip1, Cyclin D1 (CCND1), Cyclin A2 (CCNA2) and Cyclin E1 (CCNE1) in LNCaP (upper panel) and C4-2 (lower panel) cells in the absence (white bar) and presence (gray bar) of BPA. For the previously mentioned experiments, significance was determined using the Student’s t-test compared to 0 h or vehicle treatment (*P< 0.05, **P ≤ 0.01, ***P ≤ 0.001). Bars, s.e. of three independent experiments.
-
Figure 4
CA in the presence of BPA and its analogues may involve ER-alpha (ESR1). LNCaP and C4-2 Cells were treated with inhibitors for ER (ICI), ESR1 (MPP), ESR2 (PHTPP), AR (bicalutamide) and GPR30 (G15) for 72 h in the presence and absence of BPA and its analogues as indicated. Cells were fixed and scored for number of cells with centrosome numbers ≥3. Significance was calculated relative to no inhibitor group. *P < 0.05, **P < 0.005, ***P < 0.001. Bars, s.e. of the three independent experiments.
-
Figure 5
BPA, BPS, DMBPA and TBBPA enhancement of centrosomal MT-aster formation is accompanied with increased expression of CEP350. LNCaP (A), C4-2 (B) cells and (C) RWPE1 cells were treated with vehicle (veh), BPA, BPS, TBBPA, DMBPA and TMBPA as indicated. Cells were fixed and immunostained for centrosomes (anti-gamma-tubulin) and MTs (anti-alpha-tubulin). Centrosomal aster formation was counted as positive if centrosomes nucleated greater than 15 asters. (A, B, C) The percent cells positive for MT-asters were scored (A’, B’, C’) Representative staining showing MT-aster formation. (D) Relative expression as determined by qRT-PCR for p150glued, EB1, Ninein and CEP350 in LNCaP and C4-2 cells in the absence (white bar) and presence (gray bar) of BPA. For above experiments, significance was determined using a Student’s t-test compared to vehicle treatment (*P < 0.05, **P ≤ 0.01, ***P ≤ 0.001). Bars, s.e.m. of the three independent experiments.
-
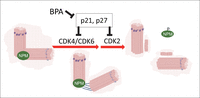
Figure 6
NPM in regulation of the initiation of centrosome duplication. Centrosome-bound NPM dissociates from centrosomes upon phosphorylation by CDK2, CDK4 or CDK6, which triggers the initiation of centriole duplication. p21Waf1 and p27kip1 inhibit CDK activation, and thus, initiation of centrosome replication.
- © 2017 Society for Endocrinology