Design and validation of specific inhibitors of 17β-hydroxysteroid dehydrogenases for therapeutic application in breast and prostate cancer, and in endometriosis
-
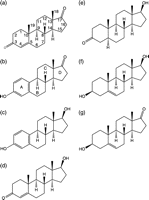
Figure 1
Steroid structures. (a) 4-Androstene-3,17-dione (Adione; with carbon positions numbered), (b) oestrone (E1; with ring positions labelled), (c) 17β-oestradiol (E2), (d) testosterone, (e) 5α-dihydrotestosterone (DHT), (f) 5-androstene-3β,17β-diol (Adiol) and (g) dehydroepiandrosterone (DHEA).
-
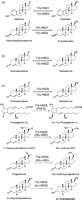
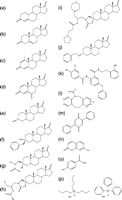
Figure 3
17β-HSD1 inhibition. (a) 3-chloroacetylpyridine-adenine dinucleotide, (b) 16α-bromoacetoxy-E2 3-methyl ether, (c) 6β-bromoacetoxyprogesterone, (d) 16,17-pyrazole-/-isoxazole-E1, (e) equilin, (f) apigenin, (g) 4-hydroxychalcone, (h) cinnamic acid, (i) EM139, (j) 6β-(thiaheptanamide)-E2, (k) EM1745, (l) 16β-m-carbamoyl benzyl-E2, (m) C5′-pyridylethylamide-16,17-pyrazole-E1, (n) STX1040 (2-ethyl-16β-m-pyridyl methyl amido-methyl-E1), (o) non-steroidal STX1040 mimic, (p) pyrimidinone core and (q) 3-benzyl-2-(2-bromo-3,4,5-trimethoxy-phenyl)-8-hydroxy-3H-benzo[4,5]thieno[2,3-d]pyrimidin-4-one.
-
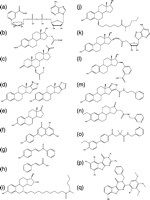
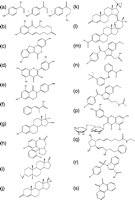
Figure 5
17β-HSD3 inhibitors. (a) 4-estrene-3,17-dione, (b) 5-androstene-3,17-dione, (c) atamestane, (d) 1,4-androstadiene-3,6,17-trione, (e) androsterone (ADT), (f) 3β-phenylmethyl-ADT, (g) 3β-amidomethyl-ADT derivatives, (h) 3-carbamate-ADT derivatives, (i) 3R-spiro-{3′-[3″-N-morpholino-2″-(3‴-cyclopentylpropionyloxy) propyl]-2′-oxo-oxazolidin-5′-yl}-5α-androstan-17-one, (j) 3-O-benzyl-androsterone, (k) BMS-856, (l) 8/9-substituted tetrahydrodibenzazocine (THB), (m) diphenyl-p-benzoquinone, (n) umbelliferone, (o) 1-(4-hydroxyphenyl)-nonan-1-one, (p) tributyltin chloride (TBT) and triphenyltin chloride (TPT).
-
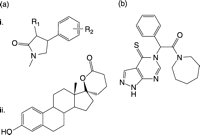
Figure 6
17β-HSD5 inhibitors and substrate analogues. (a) 1-(4′-nitrophenyl)-2-propen-1-ol to 1-(4′-nitrophenyl)-2-propen-1-one that alkylates the enzyme, (b) zearalenone, (c) coumestrol, (d) quercetin, (e) biochanin A, (f) α-methylcinnamic acid, (g) J2404, (h) cloxazolam, (i) 3α-spiro-oxirane, (j) 17β-spiro-oxirane, (k) 20α-spiro-oxirane, (l) EM1404, (m) indomethacin, (n) flufenamic acid, (o) N-(4-chlorobenzoyl)-melatonin, (p) rutin, (q) bimatoprost, (r) Ex144 and (s) 9,10-phenanthrenequinone.
- © 2008 Society for Endocrinology