Systemic therapy for neuroendocrine tumours of gastroenteropancreatic origin
- (Correspondence should be addressed to P Corrie; Email: pippa.corrie{at}addenbrookes.nhs.uk)
Abstract
Systemic therapy is one of a number of treatment options routinely used in the management of advanced, unresectable neuroendocrine tumours (NETs). In contrast to many of the other NET treatment modalities, there is at least some evidence base to justify its use. Even so, well-designed clinical trials are limited, since conducting clinical research in this complex group of rare cancers is challenging. The remit of this review article is to summarise the oncology literature and explain the role of systemic therapy in treating NETs of gastroenteropancreatic origin, identifying benefits and limitations. The molecular biology of NETs is now being unravelled, which affords new opportunities for development of mechanism-driven therapies. The rationale for some of the newer systemic targeted therapies that are showing promise in the clinic is discussed.
Introduction
Malignant neuroendocrine tumours (NETs) are rare, comprising ∼2% of all malignant tumours diagnosed in the western world. They can arise from neuroendocrine cells anywhere in the body and form a spectrum of cancers that vary in their biology, clinical behaviour, response to treatment and ultimate outcome. Oberndofer first described the carcinoid (or ‘karzinoide’) tumour in 1907, distinguishing them from other cancer types because of their slow growing nature ( Obendorfer 1907). However, it emerged that some tumours with similar histological characteristics were less indolent, and these were separated from the rest of the carcinoids, arising in the vicinity of the pancreas and thus falling into the group now identified as pancreatic endocrine tumours (islet cell tumours). Other tumours in this diverse group are NETs of unknown primary, adrenal gland tumours, poorly differentiated anaplastic small cell tumours and NETs that comprise the multiple endocrine neoplasia type I and II syndromes. The purpose of this review is to summarise the role of systemic treatments in the management of NETs occurring in the gastrointestinal tract and pancreas (GEP NETs), which are the most common sites for NETs to occur.
NETs are frequently characterised by their ability to produce peptides that can cause specific hormonal syndromes. The symptoms of carcinoid syndrome occur when the peptide, serotonin, is secreted by the primary tumour, or more usually, from liver metastases and enters the general circulation from the enterohepatic circulation. Classic symptoms include flushing, diarrhoea and bronchospasm. Less frequent but more catastrophic events include right-sided valvular heart defects, or carcinoid crisis, when large volumes of circulating vasoactive peptides generate a host of symptoms ranging from facial swelling, tachycardia and palpitations to life-threatening vasogenic shock. Since Oberndofer's first description, it has become apparent that NETs can produce a wide variety of peptides including serotonin metabolites, prostaglandins and kinins.
Endocrine tumours arising from the pancreas, also known as islet cell tumours, may produce diverse symptoms as a result of the secretion of glucagon, insulin, gastrin, corticosteroids (ACTH) or vasoactive intestinal peptide. Thus, gastrinomas can result in stomach ulcers and pain, with diarrhoea and fat malabsorption. Insulinomas produce fasting hypoglycaemia with neurological symptoms such as ataxia, confusion, weakness headache and visual symptoms. Glucagonomas can cause weight loss as a result of hyperglycaemia, with poorly healing sores and mucosal inflammation. The excess corticosteroids produced as a result of ACTH-producing tumours can cause typical Cushingoid facies, including abnormal fat distribution, reduced muscle mass, hypertrichosis, thin skin and increased risk of infection from the immunosuppressive effects of corticosteroids, as well as increased blood pressure and mental disturbances. VIPomas that release vasoactive intestinal peptide can result in flushing as well as numerous gastrointestinal symptoms including nausea and vomiting, diarrhoea, in addition to constitutional symptoms such as lethargy and weakness. In contrast to the specific symptoms generated by secretory (or ‘functional’) tumours, non-secretory (or ‘non-functional’) tumours may produce morbidity from tumour bulk and physical location.
In general, NETs are associated with a relatively inert behaviour compared with other more common epithelial cancers. However, there is a tendency to become more aggressive over time, and most patients will ultimately die of their disease. The majority of NETs (excepting insulinomas which are often benign) are malignant, and frequent sites of metastasis are lymph nodes, and liver, with less common dissemination to bone, lung and brain. The overall prognosis of NET patients differs widely according to extent of disease, histological grade and site of the primary tumour. In patients with localised NETs, the 5-year survival rates after surgery range between 60 and 90% ( Modlin et al. 2003). However, due to the indolent growth patterns of these tumours, most patients present after their disease has begun to spread, largely because of the non-specific nature of their symptoms ( Grama et al. 1992, Shebani et al. 1999). In patients with regional lymph node involvement, 5-year survival rates after surgery are between 50 and 75%, while for patients with distant metastases not amenable to surgery, around 25–40% of patients will still be alive 5 years from diagnosis ( Modlin et al. 2003).
Histopathological classification of this complex group of tumours has proven to be extremely difficult. Until recently there was no internationally approved tumour-node-metastasis (TNM) staging system for NETs, and they were staged according to their organ of origin and whether they were functional or not. Traditionally NETs have been classified according to their embryonic origin into foregut (thymus, respiratory tract, oesophageal, stomach, pancreatic, duodenal and ovarian), midgut (jejunal, ileum, appendiceal, caecal, ascending colon and from Meckel's diverticulum) and hindgut (transverse, descending and sigmoid colon) tumours. The World Health Organization (WHO) 2000 classification defined GEP NETs in a prognosis-oriented manner, taking into account not only the size of tumour, its location and presence of metastases, but also the histological grade, presence of vascular or perineural invasion, hormone production and rate of proliferation ( Solcia et al. 2000, Heitz et al. 2004). This distinguished NETs as either being well- or poorly differentiated with presence of mitoses and Ki-67 staining assists the pathologist in judging the benign or aggressive nature of the tumour ( Solcia et al. 2000, Bajetta et al. 2005, Vilar et al. 2007). When classified according to these characteristics, well-differentiated tumours are likely to remain indolent for many years, while poorly differentiated tumours behave more like adenocarcinomas, with much shorter life expectancy ( Klöppel et al. 2004, Artale et al. 2005, Bajetta et al. 2005, Panzuto et al. 2005). Thus, even in the presence of metastases, patients with well-differentiated NETs have a good prognosis, with life expectancy measured in years. Other prognostic indicators have been proposed and validated to varying extents. In the largest review of NET patients published to date (35 825 cases), Yao et al. (2008b) performed a multivariate analysis of patients with well- to moderately differentiated NETs for predictors of outcome. Statistically significant predictors were disease stage, primary tumour site, histological grade, sex, race, age and year of diagnosis.
In 2005 and 2006, the European Neuroendocrine Tumour Society (ENETS) proposed TNM systems for NETs of foregut (including pancreatic), midgut and hindgut origins at Consensus Conferences in Rome ( Rindi et al. 2006, 2007). These staging systems included a proposal for grading, in which proliferative indices were incorporated (Grade I: <2 mitosis per 10 high-power fields (HPF) and/or Ki-67<2%; Grade II: 2–20 mitosis per 10 HPF and/or Ki-67 index 3–20%; Grade III: >21 mitosis per HPF and/or Ki-67 index >20%). The prognostic relevance of the TNM staging and grading system of foregut tumours was validated in a retrospective analysis of 202 patients from a German referral centre suggesting that the new classification system could allow prognostic stratification of GEP NETs in clinical practice and research ( Pape et al. 2008). Further validation of the TNM classification for midgut and hindgut NETs by clinicopathological studies is awaited. Both the WHO and ENETS systems offer considerable value in current clinical practice.
Treatment options for NET patients
NETs vary not only in their biological and clinical characteristics, but also in their response to therapy. These factors present a huge challenge to determining the optimum treatment strategy for this diverse group of cancers ( Kerr 2006). Indisputably, the best chance of cure is surgical resection and thereafter, a variety of treatment options may be considered, however, the evidence base for treatment of NETs is remarkably poor. Despite a variety of treatments offered to the NET patients, few have been subjected to well-designed controlled trials, and therefore the true benefits of treatments in routine use are by no means entirely clear. Conducting clinical trials in the context of a rare cancer is challenging. As already emphasised, the relatively slow growth patterns of NETs raise issues in design and interpretation of the efficacy end points of early and late phase trials. The majority of those trials undertaken to date have evaluated systemic therapy, with only a handful assessing the role of other treatment modalities. Much of the published data on treatment for NETs can be criticised for being either single institution, often retrospective and assessing heterogeneous populations.
Knowledge about the tumour biology of these rare tumours is now expanding, and a host of novel small molecule drugs targeting pathways known to be up-regulated in NETs are increasing the repertoire of systemic therapeutic strategies which could potentially improve outcomes. There is therefore an imperative to ensure proper evaluation of such new treatments. Collaborative national and international research networks partnering academic and commercial enterprise is beginning to provide a platform from which a solid evidence base can be generated to allow more informed treatment decision making in the near future.
Surgery
Surgery provides the only possibility of cure, and is mostly reserved for fit patients with limited disease burden (primary and regional lymph node spread). As with other gastrointestinal carcinomas, the liver is the most common site of distant spread. However, unlike other gastrointestinal malignancies, surgical intervention is considerably more effective, and removal of both the metastasis and primary tumour is often considered if technically feasible in patients of excellent performance status, either in a synchronous or staged resection. Various groups have reported impressive 5-year survival rates up to 87% following surgical resection of NET liver metastases ( Que et al. 1995, Chamberlain et al. 2000, Nave et al. 2001, Musunuru et al. 2006). Unfortunately recurrence is observed in the majority of patients ( Cho et al. 2008). For some patients, subtotal resection of the bulk of tumour may be appropriate for palliation of symptomatic recurrence from local effects or hormone secretion ( Sarmiento et al. 2003).
Orthotopic liver transplantation is also considered an option for patients with hepatic metastatic NETs who have symptoms that are difficult to control with other therapies. Use of transplantation is in debate, however, since 62% disease-free survival at 1 year and 23% at 5 years following transplantation suggest that the short supply of livers may be more gainfully employed for conditions with better post-transplant outcomes, where 5-year survival rates in excess of 70% are observed ( Neuberger 1999, Ramage et al. 2005).
Loco-regional strategies
In patients who are not surgical candidates, regional control of liver metastases resulting in reduced tumour burden and hormone secretion has been achieved using ablative techniques such as radiofrequency ablation, laser ablation and cryotherapy. These loco-regional strategies have yet to be subjected to randomised trials, however, there are reports of promising response rates (RRs) in the range of 35–90% ( Bilchik et al. 1997). Unlike normal hepatocytes, NET liver metastases are supplied by arterial rather than portal blood supply. Therefore, hepatic arterial embolisation is a frequently used treatment for liver disease, producing RRs of over 50% ( Gupta et al. 2003). Chemoembolisation, usually using doxorubicin or cisplatin, is increasingly being employed in patients with large volume liver metastases and poorly controlled symptoms ( Kress et al. 2003, Ruutiainen et al. 2007). The optimal regimen, however, is not clear and patients undergoing these loco-regional treatments may be at greater risk of peri-hepatic sepsis or liver abscesses and infarction ( Gates et al. 1999).
Targeted radiotherapy
Patients whose tumour avidly takes up tracer as part of an octreotide scintigram may be considered for peptide receptor radionuclide therapy (PRRT). This targeted treatment modality is intuitively attractive, but resource intensive, so availability is generally confined to specialist centres with an interest in managing NETs. Furthermore, formal studies to evaluate patient benefits are sparse. Thus, key questions including identification of which patients benefit most from PRRT and at what stage in their disease it is best used remain unanswered. The optimal radionuclide is still to be determined, but treatment with 90Yttrium octreotide (90Y-DOTATOC), 90Y-lanreotide and Lu177-DOTA octreotate has been used therapeutically and reported to achieve not only tumour stabilisation but also partial responses in up to 30% of GEP NETs ( De Jong et al. 1999, Virgolini et al. 2002, Ezziddin et al. 2006, Kwekkeboom et al. 2008). Alternatively, around 20% of NETs may be identifiable on a metaiodiobenzylguanidine (MIBG) scan. 131Iodine-MIBG has been used in NETs with a positive MIBG scan, and 5-year survival of 60% and symptomatic relief in the majority of patients is described ( Kaltsas et al. 2001a, Mukherjee et al. 2001). Even so, more data are required particularly to clarify the cost–effectiveness of this approach.
Targeted systemic therapy
Targeted therapy – the focus of most modern anticancer drug development programmes, in effect already exists for NETs, by virtue of the fact that over 70% of NETs express variable levels of somatostatin receptors on their cell surface. Somatostatin receptor expression is less common in insulinomas and in poorly differentiated NETs. Synthetic somatostatin analogues have long existed and can be delivered systemically in the form of short-acting (octreotide) and long-acting (octreotide LAR and lanreotide Autogel) depot injections. The ensuing suppression of the hormonal axis can achieve significant symptomatic benefit in patients with functional tumours. Octreotide administered at doses of 100–600 μg daily in 2–4 divided doses provides symptomatic responses in up to 60% of patients and biochemical responses in up to 70% of patients, with dramatic reduction in the excreted urinary serotonin metabolite, 5-hydroxyindole acetic acid (5HIAA), occurring within weeks of commencing treatment ( Jacobsen & Hanssen 1995). Additional symptom palliation can be gained by tailoring use of other pharmacological agents, such as proton pump inhibitors for gastrinomas and diazoxide for insulinomas.
Several groups have reported anti-proliferative actions from somatostatin analogues, possibly by induction of apoptosis ( Imam et al. 1997, Ducreux et al. 2000, Aparicio et al. 2001), although significant tumour shrinkage has been reported to occur in <5% of cases ( Oberg 2001). The question whether somatostatin analogues therefore should be used in an attempt to control tumour growth and dissemination has proved controversial. However, this year the early results of the PROMID trial were released and suggest they may indeed have true antitumour activity ( Rinke et al. 2009). This double-blind trial randomised the patients with metastatic well-differentiated midgut NETs to receive either monthly octreotide LAR (30 mg) or placebo injections. Although it was planned to enter 162 patients, an interim analysis of the first 85 patients revealed that octreotide LAR significantly lengthened time to tumour progression (the primary end point of the study) compared with those receiving placebo (14.3 vs 6 months). Although there was no survival benefit in the treatment arm, a possible explanation for this was due to the low number of observable deaths. Octreotide LAR was effective in patients with both functional and non-functional NETs (over 60% of the patients had non-functioning tumours), while those with the lowest liver tumour burden (<10%) experienced the greatest benefit. This is the first trial to report a convincing disease stabilisation and will no doubt impact on standard clinical management of well-differentiated NETs in the very near future.
Systemic treatments: guided by tumour characteristics
Systemic chemotherapy, the bedrock of non-surgical oncology treatment over the last half century, represents a selective rather than specific treatment for NETs. Not all NETs respond equally to chemotherapy, therefore careful selection of patients is imperative so as to maximise the chance of response and avoid unnecessary toxicity. The degree of differentiation can determine both overall prognosis and probability of response to individual cytotoxic regimens, and therefore an accurate histological characterisation using the WHO classification and increasingly the new TNM system, plays a major role in management ( Bajetta et al. 2005). The proliferation rate, measured by the Ki-67 index, may be useful to steer medical treatments ( Vilar et al. 2007). Highly proliferative (Ki-67>20%) and poorly differentiated or anaplastic pancreatic tumours are recognised to behave aggressively, with rapid growth rate and early dissemination. However, this subtype of NET may be more sensitive to cytotoxic chemotherapy, which impairs mitosis in a greater proportion of the rapidly dividing cell population.
The foregut tumours (excluding those of thymic and bronchial origin), particularly the pancreatic NETs are most likely to respond to conventional cytotoxic chemotherapy. Midgut tumours appear often to be low proliferating tumours, and cytotoxic chemotherapy has only resulted in responses of short duration in <10% of patients. Therefore, in slow growing tumours, there is an interest to evaluate treatment modalities with mechanisms of action other than cell cycle arrest. Immune modulation using interferon-α (IFNα) administered s.c. at doses ranging 3–9 million units given 3–7 days a week has been shown to produce RRs up to 50%, measured by biochemical criteria, with durable reduction in tumour growth in up to 15% of patients and symptomatic improvement seen in 40–70% of patients ( Oberg et al. 1983). Somatostatin analogues have been used in conjunction with IFN, and long-term survival gains have been suggested ( Ducreux et al. 2000, Filosso et al. 2000, Faiss et al. 2003). However, the benefits of IFN have not definitively been shown to outweigh its toxicity so as yet, IFN is not generally adopted as routine treatment for NETs.
So-called ‘borderline tumours’ with histological evidence of a well-differentiated phenotype but moderately raised Ki-67 of 2–15% present management dilemmas that may be clarified by future correlative studies assessing proliferation indices with treatment outcomes ( Solcia et al. 2000, O'Toole & Ruszniewski 2006). In the following sections, the evidence base for treating GEP NETs with systemic chemotherapy is summarised, drawing distinction between NETs of the gastrointestinal tract (well-differentiated foregut, midgut and hindgut carcinoids), the pancreatic endocrine and anaplastic subtypes, since they appear to follow different clinical paths in terms of aggressiveness and response to cytotoxic agents.
Cytotoxic chemotherapy: when to implement it and how to assess it
In general, NETs do not show a high degree of sensitivity to chemotherapy, and this has been ascribed to low mitotic rates, presence of high levels of the anti-apoptotic protein Bcl-2 and increased expression of the multi-drug resistance gene (MDR-1; Oberg 1993, 1998, Faiss et al. 1996, Wang 1999, Kaltsas et al. 2001b). Therefore, objective RRs to chemotherapy are usually <30%. However, as previously pointed out, there is some benefit from cytotoxic therapy in a selected population of patients with more aggressive pancreatic NETs, where RRs have been reported between 40 and 70% ( Arnold et al. 2005). Systemic chemotherapy is most often employed when the NET patients present or relapse with rapidly progressive disease or the tumour has pathological features of aggressive disease and a high proliferation rate. Since most NET patients do not meet these criteria in the first instance, in order to avoid overtreatment, it is good practice in general to observe newly presenting asymptomatic patients over a 3–6-month period during which time the rate of progression can be monitored using radiological and biochemical means (NCCN Clinical Practice Guidelines in Oncology Neuroendocrine Tumours v.2.2009 www.nccn.org). In this way, some patients can be saved from unnecessary intervention, and live alongside stable disease sometimes for years, even in the presence of extensive liver metastases.
Conventionally, response to chemotherapy is defined as a measurable reduction in tumour size, using internationally agreed criteria, such as the response evaluation criteria in solid tumours criteria ( Therasse et al. 2000). However, NET patients may derive clinically significant symptom relief and biochemical reduction in hormonal secretion despite never showing objective tumour shrinkage ( Hatton & Reed 1997). Thus, response evaluation in NET studies may be either measurable change in tumour size, biochemical response or both. Whichever outcome measure is utilised, it is important to balance these potential benefits against the side effects associated with cytotoxic agents. Changes in health-related quality of life as a result of treatment need also to be assessed in order to optimise management of this diverse group of patients ( Cella 1995, Kaltsas et al. 2001b). The best validated tool for measuring patient quality of life is the European Organisation for Research and Treatment of Cancer (EORTC) QLQ C-30 instrument. This general health questionnaire is the basis upon which NET patient-specific instruments such as EORTC QLQ-GINET21 have been developed ( Davies et al. 2006).
Single agent chemotherapy for NETs
Over half a century ago, the nitrosurea compound, streptozocin (STZ) was reported to offer meaningful clinical benefits to certain pancreatic NET patients, with reported improvements in hypoglycaemic episodes and tumour load ( Table 1; Murray-Lyon et al. 1968). Of the older generation of cytotoxic DNA damaging agents, 5 fluorouracil (5FU), dacarbazine (DTIC) and doxorubicin appeared to have modest activity as single agents. Studies with 5FU report objective RRs ranging from 18 to 26% ( Oberg 1993, 1998, 1999, Moertel et al. 1994). DTIC is reported to have activity in both well- and poorly differentiated NETs. The North American Eastern Co-Operative Group (ECOG) phase II study of 42 patients with advanced pancreatic NETs reported a RR of 33%, but a median survival of around 19 months was disappointing ( Ramanathan et al. 2001). A similar trial by the South West Oncology Group reported a less impressive RR of 16% and highlighted the problem of drug-related toxicity, namely nausea and vomiting ( Bukowski et al. 1994). DTIC has also been used as second-line treatment of NETs progressing on combination chemotherapy, but a RR of just 8% ( Sun et al. 2005) suggests use in this setting cannot be justified.
Summary of selected single-agent chemotherapy trials for advanced and metastatic neuroendocrine tumours
More recently introduced cytotoxic agents have sadly proved little more effective in the treatment of advanced NETs. A phase II trial of the taxane, paclitaxel, reported a RR of 8% in 24 NET patients, associated with severe myelosuppression despite co-administered granulocyte-colony stimulating factor ( Ansell et al. 2001). A similar study of docetaxel in 21 patients yielded no objective radiological responses ( Kulke et al. 2004b), although 31% patients with raised urinary 5HIAA levels had a biochemical response. Temozolomide, an oral derivative of dacarbazine, achieved only 1 response out of 12 pancreatic NET patients ( Ekeblad et al. 2007). Capecitabine ( Krzyzanowska et al. 2006), gemcitabine ( Kulke et al. 2004a) and topotecan ( Ansell et al. 2004) have all been shown to be inactive as monotherapy in these tumours.
While most chemotherapy trials have focused on pancreatic NETs as being the potentially chemosensitive population, a recent phase II study conducted in the UK addressed whether there is a role for single agent fluoropyrimidine chemotherapy (using capecitabine) in non-pancreatic metastatic NETs. Of 19 patients recruited, 2 had a biochemical response, while 11 patients had stable disease. Time to progression was 5.3 months and overall survival was 23.7 months (Talbot & Medley, personal communication December 2009). Even so, taken together, these data suggest that single-agent chemotherapy for NETs limited benefit ( Oberg 1993, 1999). Thus, single-agent cytotoxics are rarely employed in the management of these tumours.
Combination chemotherapy for NETs
Despite low single-agent activity, combinations of cytotoxic drugs have been shown to be more beneficial to patients, with acceptable tolerance ( Table 2). Standard treatment has been largely defined by a series of relatively small randomised trials performed by ECOG in the late twentieth century. The first ECOG study combined STZ and 5FU for the first time. Compared with STZ alone, STZ plus 5FU improved RR from 36 to 63%, with superior duration of response and survival ( Moertel et al. 1980).
Summary of combination chemotherapy trials for advanced and metastatic neuroendocrine tumours
STZ-based combinations incorporating either 5FU or doxorubicin consistently generate RRs of between 40 and 60% in patients with advanced pancreatic NETs translating to a median survival of over 2 years ( Moertel et al. 1992, Gonzalez et al. 2003). ECOG went on to compare the two regimens, STZ plus doxorubicin (STZ/Dox) and STZ plus 5FU (STZ/5FU). STZ/Dox was associated with a significantly higher RR (69 vs 45%), median time to progression (20 vs 6.9 months) and overall survival (2.2 vs 1.4 years; Moertel et al. 1992). However, the doxorubicin arm was associated with considerably more drug-related toxicity, in particular, myelosuppression, emesis, nephrotoxicity and cardiotoxicity. It is worth noting that the RRs in this study are likely to be over-optimistic, since the criteria for response included clinical assessment of hepatomegaly and reduction in tumour markers rather than exclusively radiological objective response. Subsequent reports of outcomes with STZ/Dox report significantly lower RRs of 6–39% ( Cheng & Saltz 1999, Kouvaraki et al. 2004, McCollum et al. 2004). ECOG later compared STZ/5FU against doxorubicin/5FU and reported that the STZ containing regimen achieved the better survival outcome in a study which included NETs from various sites of the body ( Sun et al. 2005).
The original NET chemotherapy regimens designed by Moertel incorporated i.v. 5FU bolus injections and were associated with considerable severe drug-related toxicity, in particular, myelosuppression. From studies primarily in colorectal cancer, 5FU administered as a continuous infusion is better tolerated and achieves higher RRs compared with bolus administration ( Van Cutsem et al. 2001). A retrospective review of 15 patients treated with STZ combined with infusional 5FU ( Gonzalez et al. 2003) suggested equivalence in terms of response (53% objective response), but improved patient tolerance compared with the standard bolus 5FU-containing regimens described by Moertel et al. (1980, 1992). A more intensive regimen combining 5FU, STZ and cisplatin was developed at the Royal Free Hospital, London, and their review of 35 patients with pancreatic NETs treated with this regimen reported acceptable toxicity and a RR of 34% ( Sarkar et al. 2004).
Other combination chemotherapy regimens have been shown to achieve equivalent or better RRs in phase II studies. Capecitabine combined with oxaliplatin achieved a 30% objective RR, 20% biochemical RR and 50% symptomatic RR in 27 patients with well-differentiated NETs ( Bajetta et al. 2007). Temozolomide combined with the anti-angiogenic and immunomodulatory agent, thalidomide, has achieved RR of 45% ( Kulke et al. 2006a). Temozolomide has been reported to show in vitro synergy with capecitabine resulting in increased apoptosis of neuroendocrine cell lines, and this combination has raised interest from recent reports that an impressive RR of 71% has been observed in pancreatic NETs ( Fine et al. 2005, Strosberg et al. 2008).
Chemotherapy regimens incorporating triplet chemotherapy combinations such as DTIC, 5FU plus epirubicin, or carboplatin, gemcitabine plus irinotecan have so far not yielded significantly better results, but have been associated with unacceptable side effects ( Di Bartolomeo et al. 1995, Bajetta et al. 1998, Ollivier et al. 1998, de Lima Lopes et al. 2007). Combining chemotherapy with IFN has been tried, but has not yielded any major benefits ( Oberg et al. 1994). Given the paucity of randomised trial data other than the ECOG trials described here, STZ/5FU and STZ/Dox remain the gold standard chemotherapy regimens for unresectable pancreatics NETs in most institutions today.
Cytotoxic treatment for anaplastic NETs
Cisplatin plus etoposide, a regimen commonly used for small cell lung cancer, is widely utilised for patients with poorly differentiated NETs, based upon Moertel's study of 18 patients which reported a RR of 67% ( Moertel et al. 1991). There was far less activity in well-differentiated tumours, yielding RRs of 14% in pancreatic NETs and 0% in metastatic midgut carcinoids ( Moertel et al. 1991). A subsequent trial of 36 poorly differentiated NET patients reported a considerably lower RR of 36%, with median survival 19 months ( Fjallskog et al. 2001). Toxicity associated with the cisplatin-based regimen was significant, with nephrotoxicity being dose limiting. Therefore, today, in most cases, the second-generation platinum drug, carboplatin is substituted.
Newer chemotherapy combinations tested in this group include irinotecan plus cisplatin ( Kulke et al. 2006b) and paclitaxel, carboplatin plus etoposide ( Hainsworth et al. 2006). Indirect comparisons suggest that neither regimen is superior to standard platinum/etoposide regimens, but toxicity is problematic. The overall prognosis remains poor in this group of patients, with a 2-year survival between 20 and 33%, so there is a pressing need for identification of new therapeutic strategies.
Current chemotherapy trials
As a result of the drive internationally to improve efficacy of treatment regimens for NETs, a number of trials are investigating early activity of cytotoxic therapy in combination with other chemotherapy or with molecularly targeted agents, and these are listed in Table 3. UKINETS (formerly UK NETwork) is now an established UK society and charity and brings together specialists from all disciplines interested in research into and treatment of NETs. In 2003, it was clear that non-surgical treatment for NETs in the UK was erratic, not least because of the limited and precarious evidence base provided to clinicians upon which to base their treatment decisions. Therefore, UKINETS sets up a first UK NET trial (NET01) with the aims to 1) modernise traditional NET chemotherapy regimens, 2) identify optimal therapy and improve patient outcomes, 3) unite clinicians and systematise patient management across the UK, and 4) undertake translational research studies of pathological and molecular markers that may predict for behaviour and response to chemotherapy.
Summary of ongoing gastrointestinal tract and pancreas (GEP) neuroendocrine tumour (NET) systemic therapy clinical trials
The NET01 trial is an NCRN-approved randomised phase II study comparing two chemotherapy regimens, Capecitabine/STZ and Capecitabine/STZ/Cisplatin in unresectable metastatic NETs of the foregut and pancreas, including tumours of unknown primary site. In both arms, traditionally used i.v. 5FU is substituted with the oral 5FU prodrug, capecitabine, which has been shown to be equivalent in terms of efficacy but with improved tolerability compared with its predecessor. This study should complete recruitment by the end of 2009, and the results will be used as a basis to design future UK trials of systemic therapy for NETs.
Novel molecular targeted therapy
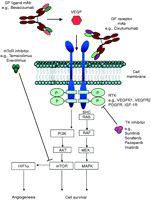
As the benefits of systemic chemotherapy have been less than conclusive across the population of NET patients, there is a strong drive to identify the specific molecular aberrations that predispose to the pathogenicity of NETs, so that they may be targeted more effectively. A detailed overview of these mechanism-driven treatments is beyond the scope of this review, however, it has been established that GEP NETs show increased expression of numerous growth factors and their receptors. Growth factors that have been implicated include vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), transforming growth factors α and β and insulin-like growth factor 1 (IGF1). Of the growth factor receptors that show increased expression, namely epidermal growth factor receptor (EGFR), PDGF receptor, IGF1 receptor and stem cell factor receptor (KIT), a number of receptors possess tyrosine kinase (TK) activity. Two key treatment strategies have been successfully implemented to date. Firstly, monoclonal antibodies (mAbs) targeting a specific ligand or receptor provide specificity against the target and are usually administered as an infusion, intermittently, every few weeks. Secondly, small molecule TK inhibitors (TKIs) are selective rather than specific to one or more TKs and can be taken orally on a daily basis ( Fig. 1).
Targeting angiogenesis appears to be an extremely successful strategy for treating a variety of cancers and a number of new anti-angiogenic agents have recently been licensed. Pancreatic NETs and carcinoids overexpress both VEGF and the VEGF receptor (VEGFR) subtypes 1 and 2 ( Christofori et al. 1995, Terris et al. 1998, La Rosa et al. 2003). A phase II trial randomised 44 patients with advanced carcinoid to receive octreotide combined with pegylated IFNa-2b, or bevacizumab, which is a humanised IgG mAb that is directed against VEGF ( Yao et al. 2008c). After 18 weeks, 95% patients receiving bevacizumab remained progression-free, compared with 68% of those in the IFNα arm. Trials are ongoing, combining bevacizumab with different chemotherapy combinations: 5FU plus oxaliplatin, capecitabine plus oxaliplatin or 5FU plus STZ in various NET patient groups (see Table 3). A new mAb, cixutumumab (IMC-A12), directed against the human type I IGF receptor (IGF1R), is now being studied in NET patients, as a significant proportion of NETs express IGF and it has been reported that high levels of IGF1R expression is associated with rapid tumour growth, increased aggressiveness and lower likelihood of cure in gastrinoma ( Furukawa et al. 2005).
Sorafenib, originally developed as a BRAF kinase inhibitor, is known to inhibit kinase activity associated with VEGFR 2, FLT3, PDGFR and FGF receptor-1 (FGFR1). Inhibition of multiple signalling pathways might be expected to be advantageous to achieving effective antitumour activity. However, early data from a phase II trial evaluating sorafenib in 93 NET patients (51 carcinoid and 42 pancreatic islet cell) reported objective RRs in only 7% of carcinoids and 11% of pancreatic NETs ( Hobday et al. 2007). This agent is currently being tested in combination with other agents such as everolimus and chemotherapy (see Table 3) in early phase trials, an attempt to improve efficacy.
Another multi-targeted TKI, sunitinib, with activity against VEGFR 1, 2, and 3, PDGFR α and β, KIT and RET, has shown impressive efficacy in advanced renal cell cancer, and it now plays a well-established role in the management of this chemoresistant tumour ( Motzer et al. 2007). Rather disappointingly, sunitinib produced only low RRs (17% of pancreatic NET patients and 2% of carcinoid tumours) in a phase II study of 109 patients with advanced NETs ( Kulke et al. 2008). Interestingly, however, the median time to tumour progression was 10.2 and 7.7 months for patients with carcinoid and pancreatic endocrine tumours respectively, suggesting a clinical benefit in terms of disease stabilisation. The clinical efficacy of sunitinib for these tumours was substantiated in a recently reported phase III randomised double blind trial that compared sunitinib against placebo in patients with progressive, well-differentiated malignant pancreatic islet cell tumours ( Raymond et al. 2009). Sunitinib resulted in a substantial improvement in median progression free survival (PFS) of 11.1 vs 5.5 months, with a hazard ratio of 0.4 in favour of sunitinib.
These data reflect the fact that TKIs may exert cytostatic effect as opposed to cytotoxic effects on tumours and emphasise the need to review the choice of end points for evaluating these agents in clinical trials. The concept of ‘disease control’ is becoming more widely accepted, whereby stable disease (as opposed to tumour shrinkage) is considered to be a positive response to treatment. Imatinib, another small molecule inhibitor of several TKs, including Bcr-Abl, PDGFR α and β and KIT, showed encouraging preclinical data inhibiting NET activity in vitro, and it has now been studied in a phase II trial of 27 advanced NET patients, where despite only 1 partial response being reported, 17 cases showed disease stabilisation ( Lankat-Buttgereit et al. 2005, Yao 2007). The anti-angiogenic TKI, pazopanib, which inhibits VEGFR 1, 2 and 3, c-kit and PDGFR, is currently being evaluated in a phase II study in patients with low- or intermediate-grade advanced NETs, underlining the ongoing interest in studying efficacy of TKIs in this disease.
The proto-oncogene Akt is overexpressed in some NETs and its downstream targets include the mammalian target of rapamycin (mTOR). This is a threonine kinase that has been implicated in NET growth via its activity on signalling pathways. In addition, mTOR-containing complexes (mTORCs) are important for hypoxia-inducible factor (HIF) synthesis and therefore mTOR inhibitors could potentially show effects on the key molecules of angiogenic signalling, the HIF proteins. The mTOR inhibitor everolimus (RAD001) has been demonstrated to abrogate NET cell line proliferation and promotes apoptosis in vitro ( Zitzmann et al. 2007). Everolimus and another mTOR inhibitor temsirolimus have now been evaluated in phase II studies in NET patients with promising early results ( O'Donnell & Ratain 2007). Everolimus has also been evaluated in combination with depot octreotide in a phase II study (US-52) of 60 NETs patient, with partial responses observed in 5 of 30 (17%) carcinoid patients and 8 of 30 (27%) patients with pancreatic NETs ( Yao et al. 2008a). A biochemical RR of 70% was noted in the 37 patients with elevated chromogranin levels.
The results of the RADIANT-1 phase II study, evaluating everolimus either alone or in combination with Sandostatin LAR in patients with advanced pancreatic NET progressing on chemotherapy, were presented this year at the 11th World Congress on Gastrointestinal Cancer, Barcelona ( Yao et al. 2010). This study enrolled patients who were resistant to prior cytotoxic chemotherapy and stratified them into two groups receiving either everolimus monotherapy if they had received prior somatostatin analogues, or combination of everolimus/Sandostatin LAR therapy. The addition of the somatostatin analogue resulted in a higher rate of disease control (tumour shrinkage plus stable disease) 84.4% compared with 77%, and improved median PFS by 7 months (16.7 vs 9.7 months). Everolimus is now being studied in combination with chemotherapy, for example temozolomide, and with other targeted agents such as sorafenib and the anti-EGFR TKI erlotinib, and data on these novel combinations are eagerly awaited.
Conclusions
Compared with the common epithelial cancers, NETs are a rare and diverse group of tumours, with varying clinical outcomes and responses to conventional chemotherapy. It appears that well-differentiated gastrointestinal NETs show little evidence of responsiveness to cytotoxic chemotherapy, and therefore it is reasonable to keep this treatment in reserve until the disease alters its clinical course to become more rapidly progressive. Pancreatic NETs, on the other hand, appear to be chemosensitive and systemic cytotoxic chemotherapy can achieve objective tumour responses in up to 60% of cases. The most commonly used regimens incorporate STZ and 5FU or doxorubicin. Anaplastic NETs show sensitivity to platinum/etoposide regimens, but overall the prognosis from these tumours is poor.
Clearly, there is a great need to improve treatment for inoperable NET patients. Better understanding of the biology of these rare and complex tumours is already beginning to drive development of novel biological therapies which show some early promise. Introduction of targeted systemic therapies, whether alone or concomitantly with cytotoxic agents, should raise the therapeutic index, maximising benefit while minimising chance of harm. It is essential for new treatment strategies to be evaluated in the context of well-designed clinical trials, which can be achieved with the good will of clinicians, scientists and Pharma to generate multinational collaborations focussing on improving outcomes associated with an orphan disease.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
- © 2010 Society for Endocrinology