n-3 polyunsaturated fatty acids and insulin secretion
-
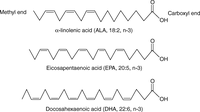
Figure 1
The structure of ALA, EPA, and DHA. ALA, EPA, and DHA contain 18, 20, and 22 carbon atoms, respectively, with two, five, and six double bonds. The first double bond from the methyl end is between the third and fourth carbon in n-3 PUFAs. ALA, α-linolenic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid.
-
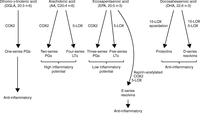
Figure 2
Bioactive mediators derived from n-3 and n-6 PUFAs. PG and LT from AA have higher pro-inflammatory potential than those from EPA and DHA. EPA and DHA are also precursors for anti-inflammatory resolvins and protectins. COX, cyclooxygenase; LT, leukotrienes; LOX, lipoxygenase; PG, prostaglandins; PUFA, polyunsaturated fatty acids.
-
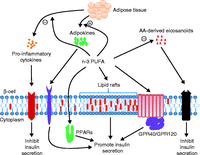
Figure 3
Summary of the potential mechanisms of n-3 PUFA-mediated promotion of insulin secretion from pancreatic β-cells. n-3 PUFAs may promote insulin secretion from pancreatic β-cells indirectly by inducing the production of the adipokines (adiponectin and eNampt) from adipose tissue, and inhibiting production of pro-inflammatory cytokines and eicosanoids from AA. Action on adipokine receptors would promote insulin secretion while the inhibitory effects of cytokine-receptor-mediated actions would be dampened. n-3 PUFAs may also directly affect β-cell function via alterations in lipid raft function, or by binding to PPARs, GPR40 and GPR120. AA, arachidonic acid; GPR, G protein-coupled receptor; PPARs, peroxisome proliferator-activated receptors; PUFA, polyunsaturated fatty acids. A full colour version of this figure is available via http://dx.doi.org/10.1530/JOE-14-0581.
- © 2015 Society for Endocrinology