The mutual dependence between bone and gonads
-
Figure 1
Gonads regulate bone physiology via the sex steroid hormones they produce. Testosterone and estrogen secreted by gonads are essential for the skeletal growth, maturation, and maintenance in both men and women. The sex steroid hormones regulate the apoptosis and/or proliferation of osteoblasts and/or osteoclasts.
-
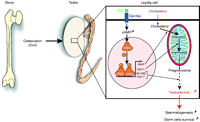
Figure 2
Bone via osteocalcin, an osteoblast-derived hormone, regulates testosterone production in testis. Following its binding to a GPRC6A expressed on Leydig cells of the testes, osteocalcin promotes in a cAMP-response element binding protein (CREB)-dependent manner testosterone production by testis. CREB binds to the promoter regions and activates the expression of several genes encoding for the enzymes that are necessary for testosterone biosynthesis, such as STAR, CYP11A, 3β-HSD, and CYP17. Steroidogenic acute regulatory protein (StAR) is crucial for transport of cholesterol to mitochondria where biosynthesis of steroids is initiated. CYP11A encodes the cholesterol side-chain cleavage enzyme (P450scc) that catalyzes the first and rate-limiting step, which converts cholesterol to pregnenolone. 3β-HSD and CYP17 encode two enzymes required during the conversion of pregnenolone to testosterone. Testosterone is a sex steroid hormone required for many aspects of testicular functions, such as germ cell survival and spermatogenesis.
- © 2012 Society for Endocrinology