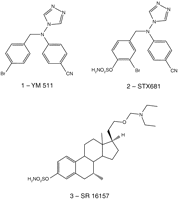
Steroid sulfatase inhibitors for estrogen- and androgen-dependent cancers
- Oncology Drug Discovery Group, Section of Investigative Medicine, Imperial College London, Hammersmith Hospital, London W12 0NN, UK
1School of Clinical and Experimental Medicine, Centre for Endocrinology, Diabetes and Metabolism, University of Birmingham, Birmingham B15 2TT, UK
- (Correspondence should be addressed to P A Foster; Email: p.a.foster{at}bham.ac.uk)
-
Figure 1
The origin of estrogenic steroids. Estrone sulfate (E1S) is found circulating at high concentrations. STS, upregulated in many hormone-dependent cancers, causes peripheral tissue conversion of E1S to estrone (E1). E1 is then reduced to estradiol (E2) by 17β-HSD type 1, binds to the estrogen receptor (ER) and causes cell proliferation. AROM, aromatase; ST, sulfotransferase; STS, sulfatase; 17β-HSD, 17β-hydroxysteroid dehydrogenase; ER, estrogen receptor; DHEA, dehydroepiandrosterone; DHEA-ST, dehydroepiandrosterone sulfotransferase.
-
Figure 2
The origin of androgenic steroids. Dehydroepiandrosterone (DHEA), synthesized from cholesterol in the adrenal gland, is sulfonated by DHEA sulfotransferase (DHEA-ST, also known as SULT2A1) to dehydroepiandrosterone sulfate (DHEAS) which is the most abundant circulating conjugated steroid. Local tissue conversion of DHEAS to DHEA is mediated by STS. Once unconjugated, DHEA is further metabolized to the active androgens, androstenediol, testosterone, and dihydrotestosterone that bind the androgen receptor (AR) leading to cell proliferation. AROM, aromatase; ST, sulfotransferase; STS, sulfatase; 17β-HSD, 17β-hydroxysteroid dehydrogenase; AR, androgen receptor.
- © 2012 Society for Endocrinology