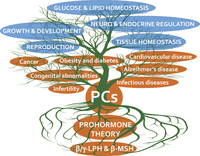
60 YEARS OF POMC: From the prohormone theory to pro-opiomelanocortin and to proprotein convertases (PCSK1 to PCSK9)
-
Figure 3
Sequence of β-LPH. Residues are indicated in color-coded circles. Those that were targeted for cleavage to generate smaller peptides for sequencing (Arg and Lys for trypsin digestion; Met for cyanogen bromide treatment) are presented in circles with wiggly borders. The pairs of basic residues flanking the sequences of β-MSH are boxed. (Data from Chrétien & Li 1967 and corrected by addition of Ile83).
-
Figure 5
(A) Biosynthesis of PC1/3. The cascade of proteolytic maturation and activation of the zymogen starts in the ER, and continues in the trans-Golgi network (TGN) and in secretory granules (SG). (B) PC1/3 and PC2 preferred cleavage sites in POMC polypeptide. The sites and the major processing are indicated by color-coded (red for PC1/3 and blue for PC2) arrowheads and lines, respectively.
-
Figure 6
LDL-C concentration in carriers and noncarriers of Q152H PCSK9 mutation in three French-Canadian families. The mutation is associated with a 37% reduction of mean plasma LDL-C or ∼1 mmol/L (P<0.001). Carriers included homozygotes (−/−) and heterozygotes (+/−) for the mutation. Their plasma LDL-C ranged from 0.5 to 3.2 mmol/L (95% CI 1.53–2.08 mmol/L), whereas that of noncarriers (+/+) ranged from 1.2 to 4.7 mmol/L (95% CI 2.39–3.32 mmol/L). Error bars represent mean±S.D., group difference determined by 2-tailed nonparametric t-test.
- © 2016 Society for Endocrinology