Novel insights into the polycythemia–paraganglioma–somatostatinoma syndrome
- Roland Därr1,
- Joan Nambuba1,
- Jaydira Del Rivero1,
- Ingo Janssen1,
- Maria Merino2,
- Milena Todorovic3,
- Bela Balint4,
- Ivana Jochmanova1,5,
- Josef T Prchal6,
- Ronald M Lechan7,
- Arthur S Tischler8,
- Vera Popovic9,
- Dragana Miljic9,
- Karen T Adams1,
- F Ryan Prall10,
- Alexander Ling11,
- Meredith R Golomb12,
- Michael Ferguson13,
- Naris Nilubol14,
- Clara C Chen15,
- Emily Chew16,
- David Taïeb17,
- Constantine A Stratakis18,
- Tito Fojo19,
- Chunzhang Yang20,
- Electron Kebebew14,
- Zhengping Zhuang20 and
- Karel Pacak1⇑
- 1Section on Medical Neuroendocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland, USA
- 2Laboratory of Pathology, National Institutes of Health, Bethesda, Maryland, USA
- 3Institute of Hematology, Clinical Center of Serbia and Medical Faculty University of Belgrade, Belgrade, Serbia
- 4Institute of Transfusiology and Hemobiology of Military Medical Academy and Institute for Medical Research, University of Belgrade, Belgrade, Serbia
- 51st Department of Internal Medicine, Faculty of Medicine, Pavol Jozef Safarik University in Kosice, Kosice, Slovakia
- 6Division of Hematology, University of Utah, Salt Lake City, Utah, USA
- 7Tupper Research Institute and Department of Medicine, Division of Endocrinology, Diabetes and Metabolism, Tufts Medical Center, Boston, Massachusetts, USA
- 8Department of Pathology and Laboratory Medicine, Tufts Medical Center, Boston, Massachusetts, USA
- 9Institute of Endocrinology, Clinical Center of Serbia, Medical Faculty, University Belgrade, Belgrade, Serbia
- 10Department of Ophthalmology, Eugene and Marilyn Glick Eye Institute, Indiana University School of Medicine, Indianapolis, Indiana, USA
- 11Department of Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, Maryland, USA
- 12Division of Child Neurology, Department of Neurology, Indiana University School of Medicine, Indianapolis, Indiana, USA
- 13Riley Hospital for Children at Indiana University Health, Indianapolis, Indiana, USA
- 14Endocrine Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA
- 15Division of Nuclear Medicine, Department of Radiology and Imaging Sciences, Clinical Center, National Institutes of Health, Bethesda, Maryland, USA
- 16Division of Epidemiology and Clinical Applications, National Eye Institute, National Institutes of Health, Bethesda, Maryland, USA
- 17Department of Nuclear Medicine, La Timone University Hospital & CERIMED & Inserm UMR1068 Marseille Cancerology Research Center, Institut Paoli-Calmettes, Aix-Marseille University, Marseille, France
- 18Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland, USA
- 19Medical Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA
- 20Neuro-Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA
- Correspondence should be addressed to K Pacak; Email: karel{at}mail.nih.gov
-
Figure 1
Ages at diagnosis of polycythemia, pheochromocytoma (PHEO)/paraganglioma (PGL) and somatostatinoma (SOM) for individual patients in chronological order. Ages at latest outpatient visit, gender, mutation status with (‡) and without (†) mosaicism of HIF2A, numbers (multiple = >3), sites (right, left) and recurrence (recur.) of lesions, presence of metastatic disease (MET) and number of surgical interventions (1st – 4th Sx, etc.) and/or radiotherapy (Rx) are indicated.
-
Figure 2
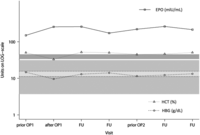
Erythropoietin (EPO), hemoglobin (HGB) and hematocrit (HCT) levels over the course of several visits for patient No. 3, starting from first presentation at NIH, before and after surgeries (prior OP1, after OP1 and prior OP2) and during intermediate follow-up visits (FU) before the second surgery at the NIH. Respective upper and lower reference intervals are indicated for EPO, HGB and HCT in grey, light grey and dark grey, respectively. EPO levels did not normalize after surgeries, strongly refuting EPO production by tumor tissue.
-
Figure 3
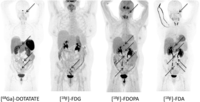
Functional imaging with [68Ga]-DOTATATE, [18F]-FDG, [18F]-FDOPA and [18F]-FDA PET/CT in patient No. 2. Radiotracer uptake is similar in [18F]-FDA PET/CT and [18F]-FDOPA, showing 5 and 6 retroperitoneal lesions, respectively. On [68Ga]-DOTATATE PET/CT only 3 retroperitoneal lesions could be identified. All of these radiopharmaceuticals showed an additional lesion at the jugular foramen (see black arrow), which was negative with [18F]-FDG. [18F]-FDG PET/CT showed only one of the retroperitoneal lesions. All retroperitoneal lesions are also indicated with black arrows.
- © 2016 Society for Endocrinology