Thyroid stem cells: lessons from normal development and thyroid cancer
- 1Department of Medicine, Mount Sinai School of Medicine, Box 1055, One Gustave L. Levy Place, New York, New York 10029, USA2Department of Molecular, Cell and Developmental Biology3Gene and Cell Medicine and 4The Leon D. Black Family Stem Cell Institute, Mount Sinai School of Medicine, New York, New York 10029, USA
- (Correspondence should be addressed to R-Y Lin; Email: reigh-yi.lin{at}mssm.edu)
Abstract
Ongoing advances in stem cell research have opened new avenues for therapy for many human disorders. Until recently, however, thyroid stem cells have been relatively understudied. Here, we review what is known about thyroid stem cells and explore their utility as models of normal and malignant biological development. We also discuss the cellular origin of thyroid cancer stem cells and explore the clinical implications of cancer stem cells in the thyroid gland. Since thyroid cancer is the most common form of endocrine cancer and that thyroid hormone is needed for the growth and metabolism of each cell in the body, understanding the molecular and the cellular aspects of thyroid stem cell biology will ultimately provide insights into mechanisms underlying human disease.
Introduction
Stem cell research in the 21st century represents an exciting area of medicine. Stem cells exhibit an extraordinary ability for self-renewal and also give rise to many specialized cells. Although significant progress has been made in generating functional neurons, cardiomyocytes, and pancreatic β cells from stem cells for use in replacement therapies for debilitating diseases such as Parkinson's disease, myocardial infarction, and type 1 diabetes mellitus, the therapeutic potential of thyroid stem cells has been largely unstudied. This discrepancy may be due to the availability of an effective, economical, standardized, and well-tolerated hormone replacement therapy for hypothyroidism. The recent identification and characterization of thyroid stem cells and the functional evaluation of thyroid cancer stem cells, however, have made significant contributions to the understanding of basic thyroid biology and thyroid cancer. Here, we review the developments that have led to our current understanding of normal and malignant thyroid stem cell biology and demonstrate how these studies provide a foundation for identification of the origin of cancer stem cells in the thyroid gland.
A stem cell overview
Definition of a stem cell
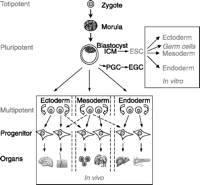
The term ‘stem cell’ is widely used to describe cells capable of both prolonged self-renewal and differentiating into one or more functional cell types (Zandstra & Nagy 2001, Gepstein 2002). Although these cells possess similar capabilities, their differentiation repertoires vary. As the cells move down the stem cell hierarchy, from zygote to fully differentiated cell, they begin to lose pluripotent capabilities and become more specialized in structure and function (Fig. 1). Stem cells are categorized by their different pluripotencies into three main groups: embryonic stem (ES) cells, adult stem cells, and fetal stem cells. Every stem cell, regardless of type, is affected by the niche, or microenvironment, in which they reside. The niche comprises both extrinsic and intrinsic signals that govern cell fate. Recent studies have described how these signals can be manipulated to direct the in vitro differentiation of both adult and embryonic signals into a number of advanced cell lineages (Keller 1995, Barrilleaux et al. 2006).
The scheme demonstrates the hierarchy of stem cells. A totipoptent stem cell, such as a zygote, can give rise to all of the cell types in an entire body as well as the cell types that make up the extraembryonic tissues such as the amnion, the chorion, and the placenta. A pluripotent stem cell, such as an embryonic stem cell, can give rise to all of the cell types in the body but they cannot make extraembryonic tissues. A multipotent stem cell is a stem cell that has the ability to develop into more than one cell type. ICM, inner cell mass; ESC, embryonic stem cell; PGC, primordial germ cell; EGC, embryonic germ cell. (From Wobus & Boheler 2005, used with permission).
ES cells
The capacity of a zygote to generate an entire organism, called totipotency, is retained up to the eight-cell stage of the morula (Wobus & Boheler 2005; Fig. 1). Subsequent formation of the blastocyst results in the formation of an outer trophoblast layer of cells surrounding a core of cells referred to as the inner cell mass. Cells of the inner cell mass are no longer totipotent, but retain the ability to develop into variable cell types of the embryo. The first mouse ES cell lines were derived from the inner cell mass by two independent laboratories in 1981 (Evans & Kaufman 1981, Martin 1981); nearly 17 years later, in 1998, the first human ES cells were derived from human blastocysts (Thomson et al. 1998). ES cells exhibit great plasticity and are able to self-renew. In vitro differentiation of ES cells requires the formation of cell aggregates referred to as embryoid bodies. The embryoid bodies display regional expression of embryonic markers specific to ecto-, meso-, and endodermal lineages (Gepstein 2002). Exposure to a cocktail of variable growth factors and hormones at adequate dosages and appropriate times allows for the differentiation of ES cells toward various lineages, including cardiomyocytes, pancreatic β cells, hematopoietic progenitors, hepatocytes, neurons, and thyroid follicular cells (Kennedy et al. 1997, 2007, Keller & Snodgrass 1999, Lumelsky et al. 2001, Boheler et al. 2002, He et al. 2003, Nishimura et al. 2003, Ku et al. 2004, Kubo et al. 2004, Shirahashi et al. 2004, Foshay et al. 2005, Ogawa et al. 2005, Arufe et al. 2006, Jiang et al. 2007).
Adult stem cells
Recent ethical concerns and debates over the use of ES cells have driven the search for an alternate, comparable source of pluripotent stem cells. Most organ systems include adult stem cells, which also possess self-renewal capabilities. However, adult stem cells are limited in their differentiation potential (Lowry & Richter 2007). These cells are therefore deemed ‘multipotent’ – they retain the potential to differentiate into only the cell types specific to the tissue or organ in which they reside (Fig. 1). Adult stem cells are critical for normal replacement and repair of tissues damaged by disease or injury. They have been found to exist within bone marrow, skeletal muscle, adipose tissue, the central nervous system, and others (Barrilleaux et al. 2006).
Fetal stem cells
Another alternative to ES cells, although no less controversial, are fetal stem cells. In general, stem cells are present in a variety of fetal tissues, including cord blood and most fetal organs. Their pluripotent properties range between those of embryonic and adult sources; they tend to be more primitive the earlier in gestation they are derived (Guillot et al. 2006). It has been suggested that fetal stem cells are more plastic than adult stem cells. Hematopoietic stem cells from fetal blood proliferate more rapidly than those found in cord blood or adult bone marrow (O'Donoghue & Fisk 2004). Furthermore, fetal mesenchymal stem cells escape recognition by alloreactive T-cells when cultured with peripheral blood lymphocytes, indicating that these cells are less immunogenic than adult stem cells and may therefore be more therapeutically useful (Gotherstrom et al. 2004).
ES cell-derived thyroid follicular cells
In vitro derivation of thyroid follicular cells from murine ES cells
Many culture conditions and selection strategies for the generation of highly enriched, lineage-specific populations from ES cells have been described. Recently, we reported a stepwise protocol by which we derived thyrocyte-like cells from the wild-type murine CCE ES cell line. Briefly, ES cells are allowed to aggregate and form embryoid bodies in the presence of serum. They are then treated for 2 weeks with thyroid-stimulating hormone (TSH), the main regulator of the thyroid gland (Lin et al. 2003, Lin & Davies 2006). Six-day-old ES cell-derived embryoid bodies treated in this manner express genes traditionally associated with thyroid follicular cells: pair box gene 8 (PAX8), the Na+/I− symporter (NIS), thyroglobulin (Tg), thyroperoxidase (TPO), and the TSH receptor (TSHR; Lin et al. 2003). The embryoid bodies also exhibited thyroid-specific function, such as cAMP generation in the presence of TSH (Lin et al. 2003). These attributes suggest that thyrocyte development is at least partly recapitulated in this ES cell model system, and that it is possible to study the precise effects of various growth factors or signaling pathways on ES cell differentiation.
Despite these successes, this strategy produced only variable and transient thyrocyte-like cells, which were neither pure nor present in sufficient quantities for additional functional studies. It is apparent that protocols need to be optimized to allow the efficient and reproducible differentiation of ES cells into mature thyroid cells and that selection strategies are needed to enable the tracking and isolation of a highly pure cell population throughout the process. We must also ensure that any mature thyroid cells generated from this protocol display thyroid-specific functional properties, such as the synthesis of Tg, the transportation of iodide, the iodination of Tg, and the storage and release of thyroid hormones in a physiologically appropriate manner, both in culture and after transplantation into an animal model.
We have learned that it is necessary to genetically manipulate specific thyroid proteins to enable the sustained production of functional thyroid cells in this system. TSHR, a thyroid cell-surface protein expressed in early embryoid bodies, could be an ideal marker for use in cell sorting to isolate thyroid-specific lineages during this process. More importantly, the fact that TSHR knockout mice are severely hypothyroid and have a poorly developed thyroid gland implies that both TSH and TSHR are important for embryonic thyroid development. Therefore, we developed a novel three-stage induction method based on the use of a green fluorescent protein (GFP)–TSHR fusion protein to enrich the proportion of thyroid cells obtained during ES cell differentiation and to allow us to easily track thyroid-specific development in ES cells in vitro. Our modified approach consists of incubation of ES cells engineered to express the fusion protein with TSH followed by enrichment of TSHR-expressing cells in early embryoid bodies by GFP-based cell sorting. Finally, the enriched cells are matured in DMEM serum-free medium supplemented with TSH. We observed that these ES cell-derived GFP-positive cells form thyroid follicle-like cell clusters on Matrigel (Arufe et al. 2006). Immunofluorescent studies confirmed the co-localization of TSHR with NIS in the clusters and indicated that NIS expression is confined to the plasma membrane. Furthermore, active I− uptake by these cells implies that the NIS seen in the immunofluorescent studies is functional (Arufe et al. 2006). Our findings indicate that the generation of a TSHR-based selectable marker is a good strategy that enables the efficient and reliable isolation of thyroid lineage progenitors from ES-cell cultures in the presence of TSH.
Although these findings are encouraging, several issues remain to be resolved regarding the maturation of thyroid cells in these cultures. First, the differentiated cell populations are still heterogeneous; the percentage of thyroid cells (<1%) remains too low to allow detailed characterization. Secondly, as with many other ES cell-derived cell-specific lineages, characterization of pure, lineage-committed stem cell populations is still problematic. Finally, it is not yet possible in our model to make perfect thyroid cells. For example, no Tg-expressing cells were observed in our cultures and in vivo transplantation studies were not performed. More effective approaches are required for generating functional, ES cell-derived Tg-producing cells capable of rescuing the hypothyroid phenotype in an animal model.
If the ES cell model is to be used as a valid model of thyroid embryogenesis and, ultimately, a source of thyroid cells, the differentiation protocol should be designed to mimic thyroid development. In vitro manipulation of mouse ES cells has allowed for development of such a mouse model for thyroid embryogenesis. This model has been used to identify a set of markers that can differentiate between primitive and fully functional thyroid cells (Lin et al. 2003, Arufe et al. 2006, Lin & Davies 2006). According to this model, the thyroid transcription factors, TTF1, TTF2, and Pax8 are expressed upon the onset of thyroid development, when the thyroid begins to bud from the floor of the primitive pharynx. These markers are likely indicative of thyroid stem cells or progenitor cells. By mouse embryonic day 14, lineage-restricted thyroid precursor cells begin to express TSHR, and by day 15.5, fully differentiated thyroid follicles begin to express the thyroid differentiation markers Tg, TPO, and NIS (Van Vliet 2003, Davies et al. 2005). A similar model for human thyroid embryogenesis has been proposed that differs primarily in timing. Using these models, and noting the expression of early and late thyroid markers, thyroid stem cells can be isolated and expanded for potential therapeutic applications.
Induction of thyrocyte progenitors from human ES cell-derived endoderm
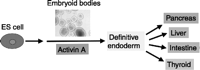
In order to elucidate the mechanisms of thyroid embryogenesis during human development, a human embryonic model is currently being developed. As with murine cells, the manipulation of the pluripotent potential of human ES cells in vitro may allow for their directed differentiation towards the thyroid lineage under appropriate conditions. Recently, D'Amour et al. (2005) demonstrated that activin A, a member of the TGFβ family, stimulates the differentiation of human ES cells into up to 80% definitive endoderm cells. Their data suggested that the formation of endoderm from differentiating human ES cell cultures is similar to the differentiation of endoderm that occurs during vertebrate gastrulation in vivo. Subsequent transplantation of these human ES cell-derived endoderm cells under the renal capsule causes them to further differentiate into more mature cells of endodermal organs (D'Amour et al. 2005). Several other groups have reported an ability to generate a variety of endoderm-derived cells from differentiating human ES cells, including pancreatic β cells and hepatocytes (Fig. 2; Kubo et al. 2004, Jiang et al. 2007, Mfopou et al. 2007). Given the fact that thyroid follicular cells also arise from the endoderm during vertebrate development, it is likely that similar strategies could be used to stimulate their differentiation from human ES cells. At present, human ES cell-derived, endoderm-positive cells have been shown to express several early thyroid markers, including TSHR and Pax8 (Thomas & Lin unpublished observations). However, because Tg is not readily expressed in these cells, current conditions must be optimized to allow for in vitro differentiation of human ES cells into functional thyroid cells.
Adult stem cells in the thyroid gland
The existence of thyroid stem cell populations within the mature thyroid was first postulated by Dumont et al. (1992), in part because the growth of thyroid transplants in recipient animals requires the injection of a minimum number of cells and also because foci formation in cloning assays is very inefficient (Watanabe et al. 1983, Dumont et al. 1992). It has since been proven that a population of adult stem cells co-expressing the pluripotent marker Oct-4, the endodermal markers Gata-4 and HNF4α, and the TTF Pax8, exists within human goitrous thyroid (Thomas et al. 2006). These findings reinforce the hypothesis that a subpopulation of pluripotent stem cells exists within goitrous thyroid (Thomas et al. 2006, Hoshi et al. 2007). Thyroid stem cells isolated from primary cultures derived from human goiters can differentiate into thyroid cells in vitro (Lan et al. 2007). Stem cells were isolated from primary thyroid cultures and grown either as a monolayer or embedded in collagen. Cells induced with TSH in serum-enriched medium expressed Pax8, Tg, NIS, TSHR, and TPO mRNA (Lan et al. 2007). Furthermore, differentiated cells embedded in collagen can take up iodide when stimulated with TSH (Lan et al. 2007).
Side populations of cells exhibiting characteristics of stem or progenitor cells have also been identified in normal mouse thyroid (Hoshi et al. 2007). Side populations with characteristics of hematopoietic stem cells were first identified in bone marrow cells through the use of the dye Hoechst 33342 (Goodell et al. 1996). Gene expression profiles indicate that side-population cells in the thyroid express limited levels of thyroid differentiation marker genes, including TPO and Tg. Instead, they express genes related to stem cell pluripotency and self-renewal (Hoshi et al. 2007). Although it has been postulated that a subset of thyroid stem cells, estimated at most to be 1 of 1000, are sufficient to replenish the pool of fully differentiated thyroid cells, there is currently no direct proof of this hypothesis. However, the findings of side populations in adult thyroid certainly provide a new direction of research. It will be important to demonstrate that these side-population cells are able to self-renew and to differentiate into thyroid cell lineage, and that, when transplanted into a hypothyroid animal model, these cells can actively participate in the regeneration of thyroid cells.
Theory of cancer stem cells
Cancer is characterized by mutations that cause uncontrolled cell proliferation and the formation of tumors. Although the vast majority of these mutations activate cell cycle checkpoints that curtail hyperproliferation, there are instances in which cells escape these checkpoints and develop into cancer. Some evidence suggests that a small population of tumor cells have stem cell like properties. This has lead to the evolution of the cancer stem cell hypothesis. This theory states that tumors both initiate and are maintained by this small population of cancer stem cells. It is uncertain whether these cells are actually stem cells, or if they are formerly normal cells that have obtained stem cell like properties. If these cells do originate from stem cells, it will be important to determine whether they are stem cells or progenitor cells.
The cancer stem cell hypothesis arises in part from the observation that cancer cell populations are not homogenous. In 1971, Park et al. (1971) was able to show that although tumors arise from a single cell, the cells that constitute the tumor are not identical to one another. Evidence of this heterogeneous population led others to investigate whether some cancers exhibit forms of cellular hierarchy. In acute myeloid leukemia, Hope et al. (2004) performed serial transplantation experiments of leukemic cells from the bone marrow or peripheral blood of patients into severe combined immunodeficiency (SCID) mice and identified what they classified as three types of stem cells: short-term, long-term, and quiescent long-term SCID leukemia-initiating cells (Hope et al. 2004). These cells were able to repopulate secondary mice at various time points, indicating that these cancer cells had self-renewal properties similar to those of stem cells.
Side populations of cells with stem cell properties have been identified in many cancers (Kondo et al. 2004, Patrawala et al. 2005, Chiba et al. 2006). A side population was identified among thyroid cancer cell lines for the first time in 2007; 0.25% of cells in the anaplastic thyroid carcinoma cell line were determined to be side-population cells. Other thyroid cancer cell lines harbor an even smaller percentage of side-population cells (0.1%; Mitsutake et al. 2007). Moreover, a differential gene expression profile between side-population cells and non-side-population cells was revealed by comprehensive analysis of gene expression using microarray chip. Particularly, several genes related to stemness (ABCG2, MYC, JUN, FZD5 HES1, and JAG1) were up-regulated in side-population cells. However, both side-population and non-side-population cells derived from these thyroid cancer cell lines can form tumors when injected into nude mice (Mitsutake et al. 2007). This finding suggests that cancer stem cells are not exclusive to or identical to side-population cells.
The cancer stem cell theory couples the idea that stem cells are responsible for cancer with the hypothesis that distinct mutations in signaling pathways are involved in tumorgenicity. Signaling pathways associated with ES cell proliferation and differentiation are particularly important to the cancer stem cell theory. For example, the Wnt/β-catenin pathway is involved in the maintenance and self-renewal of hematopoietic stem cells and progenitor cells; overexpression of WNT protein is also seen in numerous human cancers (Taipale & Beachy 2001). Sonic hedgehog, which is involved in maintaining hematopoietic stem cells and progenitor cells as well as brain development, is associated with brain tumors (Ruiz i Altaba et al. 2002).
Recently, much attention has been given to p63, a homolog of the tumor suppressor p53. Preto et al. (2004) found p63 in the main cells of the solid cell nests, but no trace of p63 in C cells. Cameselle-Teijeiro et al. (1995) reported that the main cells of solid cell nests of the human thyroid gland have some stem cell properties, including a capacity for self-renewal and an ability to differentiate into more than one cell type. However, these cells do not express terminal thyroid differentiation markers such as Tg and calcitonin. The researchers suggested a possible link between papillary oncogenesis and the existence of stem-like cells in the adult thyroid. This view is shared by Burstein et al. (2004) who proposed a role for p63 in a stem cell model of papillary carcinoma. p63 has been detected via immunohistochemistry in basal and parabasal squamous cells and in bronchial basal cells of squamous and bronchial epithelia (Yang & McKeon 2000, Pellegrini et al. 2001). These investigators showed that p63 expression in solid cell nests and papillary thyroid carcinoma cells closely resembles the stem cell-associated p63 staining patterns reported in squamous and bronchial epithelia. They therefore suggest that p63 may be ‘the cell origin of papillary carcinoma.’ However, it remains to be proven whether p63 is actually involved in the tumorigenic process.
Cellular origin of thyroid cancer stem cells
Thyroid carcinoma is the most common type of endocrine cancer. The American Cancer Society estimates that there will be ∼33 550 new cases of thyroid cancer diagnosed in the United States in 2007. There are four main types of thyroid cancer: papillary, follicular, medullary, and anaplastic. Papillary carcinoma, the most common form, is derived from thyroid follicular cells. Follicular carcinoma is the next most prevalent form. Both papillary and follicular carcinomas of the thyroid have a very good prognosis. Medullary carcinoma forms in the C cells of the thyroid gland and has usually spread to other organs by the time of diagnosis. Anaplastic carcinoma is the most deadly form of thyroid cancer, with a mean survival rate of only 8 months after diagnosis, according to the American Cancer Society (Cancer facts and figures 2007, available at http://www.cancer.org/docroot/stt/stt_0.asp).
The thyroid cancer stem cell hypothesis holds that thyroid cancer stem cells originate either from normal stem cells, progenitor cells, or more mature cells that have dedifferentiated. Although any of these origins is possible, most researchers believe that stem cells or progenitor cells are the most likely culprits. Cancer progression requires that cells overcome the barrier that somatic cells have in regard to proliferation, and the lifespan of differentiated cells is too short to obtain all the mutations associated with cancer. The primary evidence for this theory is that cancer populations are not homogenous. Numerous studies have shown that only a subset of cancer cells – those with properties of stem cells – are tumorigenic. For example, Al-Hajj et al. (2003) showed that breast cancer cells can be separated into tumorigenic and non-tumorigenic populations based on specific markers. In particular, the CD44+CD24/low lineage populations can reconstitute tumors that are made up of a heterogeneous population of cells similar to that from which they were obtained. Subsequently, Zhang et al. (2006) presented a model for the origin of the four types of thyroid carcinomas based on their differentiation levels. Anaplastic cancer could form directly from a stem cell because it is poorly differentiated. Follicular and papillary carcinomas, which are well-differentiated, could arise from bi-potential stem cells. Meduallary carcinoma, another well-differentiated cancer, could originate from progenitor C cells (Zhang et al. 2006). Although the discovery of stem cell markers in the thyroid gland indicates the potential for the existence of thyroid cancer stem cells, the identification of these cells could prove to be quite difficult due to the extremely low lifetime turnover in the thyroid gland.
The cellular origin of anaplastic carcinoma is of special interest because no successful treatment for it exists. The classical view for its origin is that it results from additional mutations to papillary carcinomas. However, while papillary carcinomas are marked with rearrangement of the RET gene, these mutations are not generally found in anaplastic carcinomas. To date, there has been no confirmation that anaplastic carcinoma does result from additional mutations to papillary carcinomas. Takano & Amino (2005) use this evidence to support their hypothesis for the origin of anaplastic carcinoma. Their point of view is that anaplastic carcinoma forms from the remnants of fetal thyroid cells, instead of normal thyroid follicular cells, before adolescence, and already have cancer properties prior to the onset of their division. Furthermore, the fetal cell carcinogenesis hypothesis suggests a similar gene expression profile between fetal thyroid cells and thyroid cancer cells. However, this hypothesis needs further investigation before it can be considered as an explanation for anaplastic carcinoma.
Clinical implications of thyroid cancer stem cells
If proven to be true, the cancer stem cell theory could profoundly affect how cancer is treated. The identification of cancer stem cells will provide a specific target for chemotherapy and drugs, and it may even dictate the aggressiveness of the treatment. Currently, many common chemotherapy and radiation protocols target all dividing cells, regardless of whether or not they are cancerous. If, however, the disease is due to cancer stem cells, this could be the wrong approach. If the cancer stem cells are quiescent when therapy is started, they may survive treatment intended to kill dividing cells. In this case, the patient may appear to have made a full recovery only to relapse years later when the cancer stem cells are reactivated. If this scenario is true, it is possible that today's cancer therapies are merely helping patients ‘maintain’ their lives rather than getting to the root of the problem: cancer stem cells. Development of drugs that specifically target cancer stem cells may render cancer treatment more successful and efficient, and less toxic to the patient. For example, stem cells have active ATP-binding cassette transporters of specific types, which may increase the resistance of cancer stem cells to typical treatment options. Stem cell-specific targets such as these could be very useful in the design of new therapies. Although the cancer stem cell hypothesis is still being worked out, the implications for future therapies are great, and it may even be able to change the way we diagnose and treat the disease.
Concluding remarks
The studies reviewed here highlight recent successes in the generation of thyroid cells from ES cells and provide evidence for the presence of adult stem cells in human and mouse thyroid glands and of cancer stem cells in thyroid cancer. It also emphasizes future challenges in thyroid cancer therapy. Future work will focus on the molecular and the cellular mechanisms that occur during ES cell-based thyroid development, and on the progression of adult thyroid stem cells to differentiated thyroid cells and ultimately to thyroid cancer. The continued application of lessons from stem cell research to many different areas of biology and medicine will no doubt increase understanding of the cellular basis of normal and malignant thyroid stem cell development. It will also contribute critical insights to the development of more efficacious disease-fighting therapies.
Acknowledgements
We would like to thank Drs Terry Davies and Gordon Keller and members of their laboratories for excellent technical assistance. This work was supported in part by the NIH National Institutes of Health Grant R01 DK068057 (to R-Y Lin). The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
- © 2008 Society for Endocrinology