Mouse models of transforming growth factor β impact in breast development and cancer
- R Serra and
- M R Crowley
- Department of Cell Biology, University of Alabama at Birmingham, 1918 University Boulevard, Birmingham, AL 35294-0005, USA
- (Requests for offprints should be addressed to R Serra; Email: rserra{at}uab.edu)
Abstract
It is now recognized that transforming growth factor β (TGF-β) is an important factor that regulates normal breast development as well as breast cancer. Genetically engineered mouse models have been used to determine the role and mechanism of TGF-β action in normal development and diseases of the breast. Using these models, it has been determined that TGF-β regulates many steps of normal mammary gland development including branching morphogenesis, functional differentiation, cell-lineage decisions, and involution. Effects of TGF-β on normal development are mediated through signaling in both the epithelial and stromal compartments. In cancer, mouse models have indicated that TGF-β has biphasic effects on tumor progression, acting as a tumor suppressor in early stages of cancer and promoting invasion and metastasis at later stages. In addition, TGF-β may play a role in tumor progression through effects on the microenvironment. Recently, experiments in several mouse models have suggested that antagonism of TGF-β signaling may provide a therapeutic target for late-stage breast cancer, blocking metastasis without detrimental side effects. In the future, genetically altered mice will be used to establish models of human breast disease providing opportunities to test strategies for disease prevention and treatment.
(Present address of M R Crowley is Department of Genetics, University of Alabama, Birmingham, 720 20th Street South, Birmingham, AL 35294-0024, USA)
Introduction
Development of the mouse mammary gland begins at embryonic day 11 (E11) as an invagination of the ectoderm into the underlying ventral mesoderm (Imagawa et al. 1994, Hennighausen & Robinson 2001, Silberstein 2001, Parmar & Cunha 2004). A prominent mammary bud surrounded by condensed mammary mesenchyme is evident by E15. The mammary bud elongates slightly into the primitive mammary fat pad resulting in a mammary anlagen that consists of 12–15 branches at birth. The anlagen grows isometrically with the mouse until puberty at which time ovarian hormones stimulate the formation of large terminal end buds (TEBs) that rapidly invade the fat pad. Eventually, the TEBs reach the limits of the fat pad and regress. The adult female mammary gland then undergoes limited rounds of proliferation, differentiation, and apoptosis in response to hormonal changes of the estrus cycle. Pregnancy induces the differentiation of alveolar milk producing cells and lobuloalveolar structures along the existing ductal tree that eventually fill the fat pad with epithelium. Lactation signals a fully functional mammary gland and the differentiated cells are capable of producing and secreting milk. After weaning, milk stasis results in a process called involution. Involution is a two-step process that first involves programmed cell death of the milk-producing cells followed by extensive matrix remodeling, returning the mammary gland to a quiescent state. Many of the highly orchestrated biological processes that occur during the cycle of normal mammary gland development, including cell growth, invasion, differentiation, and apoptosis, are deregulated during the formation and progression of mammary cancer.
The transforming growth factor β (TGF-β) superfamily is recognized as a large and evolutionarily conserved family of secreted multifunctional peptides involved in regulating almost every aspect of cellular behavior (Miller et al. 1990, Roberts & Sporn 1990, Kingsley 1994, Massague et al. 2000). TGF-β is the prototype for this large peptide family. Members of the superfamily include three isoforms of TGF-β (TGF-β1, TGF-β2, and TGF-β3): the activins and inhibins; the growth and differentiation factors (GDFs); and the bone morphogenetic proteins (BMPs). TGF-β is first synthesized as a precursor protein with a signal sequence and a large prodomain (Pircher et al. 1986). TGF-β is secreted in an inactive, latent form consisting of a 25 kDa mature peptide in a non-covalent association with the N-terminal prodomain of the precursor protein (latency-associated peptide; Wakefield et al. 1989). Activation of the latent form of TGF-β may be a major regulatory step in controlling TGF-β function in vivo (Barcellos-Hoff 1996, Murphy-Ullrich & Poczatek 2000, Koli et al. 2001). A constitutively active form of TGF-β can be produced by mutations in cysteine residues 223 and 225 (Tgfb223/225) that abrogate the ability of latency-associated peptide to associate with the mature peptide (Brunner et al. 1989).
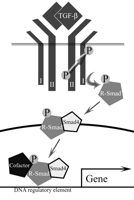
TGF-β signals through a heterotetrameric receptor complex composed of type I (TβRI) and type II (TβRII) serine threonine kinases (Fig. 1⇓; Derynck 1994, Ten Dijke et al. 1996, Massague 1998b). TGF-β ligand binds to TβRII on the cell surface and recruits TβRI into the heterotetrameric complex (Wrana et al. 1994). TβRII, a constitutively active kinase, phosphorylates and activates the kinase function of TβRI. Downstream substrates of TβRI transduce the signal to the nucleus. The most well-characterized TGF-β signaling molecules are the Smads (Derynck & Zhang 1996, Massague 1998a, Massague & Wotton 2000, Wrana & Attisano 2000). Receptor-associated Smads (R-Smads) interact with and are directly phosphorylated by TβRI. Phosphorylated R-Smads can associate with Smad4, translocate to the nucleus, and regulate transcription either alone or in combination with transcriptional co-activators and co-repressors from other signaling cascades. In this way TGF-β signaling is integrated with signaling from other growth factors (Zhang & Derynck 1999, Attisano & Wrana 2000, Massague & Chen 2000). Specific R-Smads transduce distinct signals for members of the TGF-β superfamily. Smad2 and Smad3 mediate signaling by TGF-β and activin whereas Smad1, Smad5, and Smad8 mediate BMP signaling. Inhibitory Smads (I-Smads), Smad6 and Smad7, have also been identified (Hayashi et al. 1997, Hata et al. 1998). TGF-β receptors and Smads have been identified as potential tumor-suppressor genes in humans (Markowitz et al. 1995, Eppert et al. 1996, Hahn et al. 1996, Serra & Moses 1996, Massague et al. 2000, Derynck et al. 2001).
TGF-β signaling pathway. TGF-β binds to the type II receptor (II) on the cell surface. The type II receptor associates with and phosphorylates the type I receptor (I). The type I receptor phosphorylates receptor-associated Smads (R-Smads), which form a complex with Smad4, translocate to the nucleus, and act as transcription factors in combination with a variety of co-factors. Dominant-negative forms of the type II receptor lack kinase activity, preventing phosphorylation and activation of type I receptor and subsequent signaling events.
Mouse models of TGF-β in mammary development
Members of the TGF-β superfamily, their receptors, and signaling molecules are expressed and play critical roles in every phase of post-natal mammary gland development (reviewed in Daniel et al. 2001 and Serra & Crowley 2004). In situ hybridization analysis of developing mouse mammary glands with probes specific for the different TGF-β isoforms demonstrated overlapping patterns of expression in the TEBs as well as the growth-quiescent ducts (Robinson et al. 1991). Expression levels were increased during pregnancy but dropped dramatically during lactation (Robinson et al. 1991). The message for TGF-β3 increased 6 h after the cessation of lactation (Nguyen & Pollard 2000) followed by increases in TGF-β1 and -β2 that peaked between 4 and 6 days of involution (Faure et al. 2000, Strange et al. 1992). Localization of TGF-β proteins in the mouse mammary gland has also been characterized (reviewed in Ewan et al. 2002). TGF-β proteins were localized in distinct and overlapping patterns in both epithelial and stromal compartments. TβRI and TβRII have also been localized in the mouse by in situ hybridization and immunohistochemistry to both the epithelial and stromal compartments during all phases of mammary gland development (Joseph et al. 1999).
The expression pattern of TGF-βs in the mouse suggested that TGF-β could have roles in regulating branching morphogenesis, lactation, and involution. Experiments using mouse models have confirmed a role for TGF-β in several steps of post-natal mammary gland development (Table 1⇓; Fig. 2⇓). The growth-suppressive effects of TGF-β on the TEBs were first demonstrated by implantation of slow-release pellets containing active TGF-β1 or TGF-β3 in the mammary fat pad in front of the elongating ductal tree (Silberstein & Daniel 1987, Robinson et al. 1991). Whole-mount and histological analysis of control mammary glands implanted with pellets containing BSA showed large, multilayered TEBs with high rates of proliferation. In contrast, the TEBs in glands implanted with beads containing TGF-β regressed and had proliferation rates similar to quiescent mature ducts (Silberstein & Daniel 1987, Robinson et al. 1991). The effects of TGF-β were reversible. Transgenic mice that expressed the active form of TGF-β in mammary epithelium under the control of the mouse mammary tumor virus (MMTV) promoter, MMTV-Tgfb1223/225, demonstrated a hypoplastic mammary gland (Pierce et al. 1993). That is, the ductal epithelium was delayed in filling the fat pad and virtually devoid of side branches (Pierce et al. 1993). Proliferation rates were reduced in the mammary epithelium 2-fold at 7 weeks and 4-fold at 13 weeks in the transgenic gland compared with wild-type animals (Pierce et al. 1993). Conversely, mice heterozygous for a null allele of the TGF-β1 gene (Tgfβ1+/−) have 10% of the TGF-β1 protein observed in wild-type mice. Tgfβ1+/− mice demonstrated increased ductal extension through the fat pad and increased proliferation rates of both end-bud and ductal epithelium (Ewan et al. 2002). Mice with a dominant-negative interference of TβRII function also demonstrated increased ductal extension through the end bud (Crowley et al. 2005a). The data suggest that TGF-β normally acts as an inhibitor of ductal elongation and branching. In contrast, mammary glands from Smad3-null mice demonstrated delayed ductal development and decreased side branching, most likely due to impaired ovarian function (Yang et al. 2002a). Post-natal development was normal in Smad3-null mice supplied with exogenous hormones, suggesting that signaling through Smad3 is not inherently necessary for normal mammary gland development.
Genetically engineered mouse models to determine the role of TGF-β signaling in breast development and disease
Role of TGF-β in mouse mammary development. TGF-β inhibits progression of the end buds through the fat pad during puberty (A), regulates side branching via the stroma (B), prevents formation of alveolar buds and premature milk secretion (C), and promotes apoptosis during involution (D).
Stromal–epithelial communication is critical for proper mammary gland development (Neville et al. 1998, Parmar & Cunha 2004). The stroma, composed of fibroblasts, adipocytes, lymphocytes, and other cell types, provides instructive signals that regulate epithelial behavior. Advances in manipulating mouse genetics combined with sophisticated surgical techniques have lead to the ability to evaluate signaling between stromal and epithelial compartments in the mammary gland. Several proteins, including estrogen receptor, epidermal growth factor receptor, inhibin, gelsolin, and c-Cbl, exert their effects on epithelial branching morphogenesis through the mammary stroma (Cunha et al. 1997, Robinson & Hennighausen 1997, Wiesen et al. 1999, Crowley et al. 2000, Crowley et al. 2005). Mice that express a dominant-negative form of TβRII under the control of the metallothionein promoter (MT-DNIIR) demonstrated increased ductal side branching (Joseph et al. 1999). Reciprocal transplantation experiments indicated that the side-branching phenotype was mediated through the stroma (Crowley et al. 2005). These data, in association with loss of TGF-β1 staining at developing side branches, suggest that stromal TGF-β signaling is an important regulator of ductal side branching in the mammary gland (Ewan et al. 2002). In contrast, conditional deletion of TβRII in fibroblasts using the Cre/Lox recombinase system, where Cre was under the control of the FSP1 promoter (TβRIIFspKO), resulted in reduced ductal morphogenesis and end-bud formation (Cheng et al. 2005). Defects were also observed in the composition of the stroma, with a reduction in the proportion of adipocytes to fibroblasts. Since the FSP1 promoter was activated during embryonic development, differentiation of cells in the fat pad could have been affected. It was shown previously that fat tissue is required for normal ductal outgrowth (Gregoire 2001, Couldrey et al. 2002), thus it is not clear whether the results in the conditional knockout are a direct effect of TGF-β signaling in the periductal stroma or an indirect effect of alterations in mesenchymal differentiation.
TGF-β also regulates the development and survival of alveolar epithelium. Mice in which active TGF-β is expressed under the control of the pregnancy-specific whey acid protein promoter (WAP-Tgfb1223/225) displayed normal branching morphogenesis but the development of secretory epithelium was inhibited as a result of early apoptotic death in the differentiating alveolar cells (Jhappan et al. 1993, Kordon et al. 1995, Smith 1996). Similarly, transgenic mice expressing activated TβRI in the mammary epithelium (MMTV-TβRIAAD) displayed deficiencies in late pregnancy due to increased apoptosis in alveolar structures (Siegel et al. 2003). Conversely, dominant-negative interference of TGF-β signaling in the mammary epithelium (MMTV-DNIIR) resulted in precocious alveolar development; that is, virgin mice demonstrated an early pregnant phenotype (Bottinger et al. 1997, Gorska et al. 1998). Since the phenotype was not detected until the mice were 20 weeks of age, the alveolar development seen in these mice most likely required several rounds of proliferation and apoptosis that normally occur during the estrus cycle. Accumulation of alveolar cells could be a consequence of a failure of the cells to be removed through apoptosis (Gorska et al. 1998). Crosses of MMTV-DNIIR and prolactin-null mice indicated that precocious alveolar development in the MMTV-DNIIR mice was dependent on prolactin (Bailey et al. 2004). It was suggested that prolactin and TGF-β co-ordinate alveolar development through careful regulation of apoptosis mediated by Akt. Inappropriate alveolar development has also been observed in mice expressing TβRII anitisense mRNA and in mice with Cre/Lox-mediated conditional deletion of TβRII in mammary epithelium (TβRIIMMTVKO) (Lenferink et al. 2003, Forrester et al. 2005). Likewise, the reduced levels of TGF-β1 found in Tgfβ1+/− mice resulted in an increase in alveolar development during pregnancy (Ewan et al. 2002). The data together suggest that TGF-β acts to limit inappropriate alveolar development and to regulate alveolar outgrowth during pregnancy. TGF-β’s ability to regulate apoptosis is likely to be involved.
Post-lactational milk stasis triggers the first wave of apoptosis in secretory alveoli during involution. High levels of TGF-β3 mRNA were detected in mammary gland 6 h after the induction of milk stasis (Nguyen & Pollard 2000). Transgenic mice that expressed TGF-β3 under the control of the β-lactoglobin promoter, which directs expression to alveolar epithelial cells in late pregnancy and throughout lactation, showed a significant increase in apoptotic cells at days 1 and 3 of involution compared with control, non-transgenic mice. Increased apoptosis was concomitant with Smad4 nuclear translocation and induction of phosphorylated Stat 3 (Nguyen & Pollard 2000). In addition, when mammary glands from TGF-β3-null mice, which die a few days after birth, were transplanted into syngeneic wild-type hosts, a decrease in alveolar apoptosis compared with heterozygous or wild-type glands was observed during involution (Nguyen & Pollard 2000). Furthermore, dominant-negative interference of TβRII in mammary epithelium (MMTV-DNIIR) results in a delay in involution in response to weaning or sealing the duct (Gorska et al. 2003, Bailey et al. 2004). The levels of phosphorylated Akt and Forkhead transcription factor 01 (FKHR) were increased in MMTV-DNIIR mice compared with wild-type controls, suggesting that TGF-β may act to restrain Akt and FKHR activity thereby promoting apoptosis during the initial stages of involution (Bailey et al. 2004). Together the results suggest that TGF-β3 signaling through TβRII has a key role in the early phase of involution. TGF-β3 may also play a role in re-organization of the extracellular matrix in the late stage of involution. Faure et al. (2000) determined that the primary site of TGF-β3 synthesis in late involution was myoepithelial cells. Expression corresponded to sites of laminin deposition leading the authors to propose that TGF-β3 was involved in stromal reorganization (Faure et al. 2000).
In addition to accelerated apoptosis in differentiating cells, mammary epithelial cells from WAP-Tgfb1223/225 mice demonstrated another phenotype, early growth senescence as seen by a diminished capacity of epithelial cells to repopulate a cleared fat pad (Kordon et al. 1995). Recently, a new parity-induced population of epithelial cells was discovered in the mouse mammary gland (Wagner et al. 2002, Boulanger et al. 2005). This population of cells is derived from differentiating cells during pregnancy and was identified by marking WAP-expressing cells with a lacZ reporter gene using Cre/Lox recombinase technology. It was shown that lacZ-positive cells accumulated in the mammary gland after pregnancy but did not accumulate in nulliparous mice with normal estrus cycle. The lack of persistence of lacZ-positive cells in nulliparous mice suggested that cells that differentiated during estrus were removed, most likely through apoptosis as described above. In contrast, a population of cells induced during pregnancy were maintained and contributed to future rounds of differentiation. The parity-induced cell population was shown to be pluripotent and capable of self-renewal (Boulanger et al. 2005). Experiments with mice expressing activated TGF-β1 in the Cre/Lox/Lac Z background suggested that TGF-β1 limited the self-renewal capacity of this cell population. The discovery of the parity-induced population of cells indicates that not all differentiated cells are removed during involution and explains some of the biological differences between nulliparous and parous glands.
Recently, Smad4, a central mediator of TGF-β signaling, was deleted in mammary gland epithelium using a Cre/Lox recombination strategy (Li et al. 2003). Early mammary gland development was normal; however, lack of Smad4 induced gradual increases in proliferation, alveolar differentiation, and transdifferentiation of mammary epithelium into squamous cells. Mice later developed mammary abscesses and squamous cell carcinoma. The results suggest a role for TGF-β in regulating cell-fate decisions in the mammary gland. In addition, it has been documented that 7,12-dimethybenz(a)anthracene (DMBA) treatment in mice results in the formation of a heterogeneous group of tumors, the most common being those with squamous metaplasia or adenomyoepithelial character (Cardiff et al. 1988, Rehm 1990, Bottinger et al. 1997). When mice expressing a dominant-negative form of TβRII were treated with DMBA, alterations in TGF-β signaling influenced the pathology of the tumors formed (Bottinger et al. 1997, Crowley M, Frost A, Chen D-T, Baffi M, Nicola T & Serra R, unpublished work). In wild-type mice, DMBA promoted the formation of adenomyoepitheliomas. This type of tumor was not detected in glands with altered TGF-β signaling. Furthermore, staining for αSMA and K14 was reduced or absent in glandular structures from transgenic tumors relative to wild-type tumors (Crowley M, Frost A, Chen D-T, Baffi M, Nicola T & Serra R, unpublished work). The data suggest that TGF-β is also involved in the differentiation, maintenance, and/or transformation of myoepithelial cells.
Mouse models of TGF-β in breast cancer
The growth-suppressive effects of TGF-β on epithelial cells are well documented (Alexandrow & Moses 1995). Analysis of TβRI and TβRII genes in colon and gastric cancers with microsatellite instability (RER+, replication error) provided genetic evidence of a tumor-suppressor role for TGF-β (Markowitz et al. 1995). In addition, several genes that encode TGF-β signaling molecules, TβRI and TβRII, Smad2, Smad3, and Smad4, have been found mutated or deleted in a variety of human cancers (Markowitz et al. 1995, Serra & Moses 1996, Massague et al. 2000, Derynck et al. 2001). Furthermore, mRNA levels for TGF-β signaling molecules are altered in many additional tumors. Mutations in the TGF-β signaling pathway are rare in human breast cancer; however, loss of the TβRII has been documented by immunohistochemistry (Gobbi et al. 1999, 2000). Significant loss of TβRII immunoreactivity in epithelial hyperplasia lacking atypia (EHLA) was correlated with an approximate 4-fold increase in relative risk for developing invasive breast cancer (Gobbi et al. 1999). Additionally, loss of TβRII staining was inversely correlated with tumor grade, mitotic index, and clinical stage (Gobbi et al. 2000). Several studies using transgenic mice support a role for TGF-β as a tumor suppressor in the mammary gland. Expression of transforming growth factor-α (TGF-α) in mouse mammary epithelial cells via the MMTV promoter resulted in ductal hyperplasia that progressed to carcinoma (Matsui et al. 1990). Co-expression of TGF-β1 in the mammary epithelium of the MMTV-TGFα mice significantly reduced the number of tumors that formed (Pierce et al. 1995). In addition, TGF-β expression alone (MMTV-Tgfb1223/225) inhibited mammary tumor formation in mice challenged with DMBA as compared with DMBA-treated wild-type controls (Pierce et al. 1995). In complimentary studies, dominant-negative interference of TβRII in mouse mammary epithelium (MMTV-DNIIR) increased the incidence and number of tumors that formed after treatment with DMBA (Bottinger et al. 1997). MMTV-DNIIR mice also developed spontaneous tumors with long latency (Gorska et al. 2003). Mice that express activated neu in the mammary epithelium (MMTV-neu) are a well-characterized model for mammary tumor development and progression (Muller et al. 1988). Mice develop adenocarcinomas that metastasize to the lung in a defined period of time. Mice in which the activated form of TβRI was expressed in the mammary epithelium of MMTV-neu mice developed fewer tumors with greater latency than the MMTV-neu controls (Siegel et al. 2003). Conversly, expression of a dominant-negative form of TβRII in MMTV-neu mice decreased the latency of tumor onset (Siegel et al. 2003). Conditional deletion of TβRII in mammary epithelium (TβRIIMMTVKO) also resulted in shortened tumor latency in the context of polyomavirus middle-T induced oncogenesis (Forrester et al. 2005). The data suggest TGF-βs can act as tumor suppressors in breast cancer but the mechanisms are still not clear.
One proposed mechanism involves TGF-β induced senescence in the mammary stem cell population (Kordon et al. 1995, Boulanger & Smith 2001). Innoculation of mammary glands with MMTV normally results in the development of hyperplastic alveolar nodules that presumably progress to form cancer in mice (Boulanger & Smith 2001). Introduction of MMTV into the mammary glands of 3–5-month-old WAP-Tgfb1223/225 mice showed a decrease in tumor incidence (5.8%) compared with control mice (51.7%). Epithelial cells isolated from WAP-Tgfb1223/225-expressing mammary glands also have a reduced capacity to repopulate cleared mammary fat pads compared with cells from wild-type littermates, suggesting premature senescence of stem cells in the TGF-β-overexpressing tissue (Kordon et al. 1995). Recently, a parity-induced, pluripotent, self-renewing population of cells was discovered in the mammary gland (Wagner et al. 2002). TGF-β also diminished the self-renewing capability of this cell population, raising the possibility that TGF-β may induce premature senescence in stem cells, thereby reducing the number of target cells for development of cancer (Boulanger et al. 2005).
Although there is considerable evidence indicating TGF-β functions as a tumor suppressor, there is also evidence pointing to a role for TGF-β in promoting the progression of cancer and metastasis. Many breast cancers synthesize and secrete high levels of TGF-β protein that can be detected in both the tumor cells and associated stroma (Teicher 2001). Clinical reports suggest that elevated levels of TGF-β in tumor tissue or serum of cancer patients is associated with poor prognosis (Sheen-Chen et al. 2001, Teicher 2001, Ivanovic et al. 2003). It is thought that several variables, such as tumor stage, affect the way tumor cells respond to TGF-β (Dumont & Arteaga 2000, Wakefield et al. 2000). Cells in early-stage tumors respond to TGF-β with a growth-inhibitory response, suppressing further progression of the tumor. Later, as the tumor progresses, a different genetic or epigenetic environment exists and responsiveness to TGF-β is altered so that the tumor-promoting activities of TGF-β dominate. The tumor-promoting functions of TGF-β can be a result of autocrine, paracrine, or tumor–host interactions (Dumont & Arteaga 2000). Cellular functions affected include regulation of extracellular matrix, angiogenesis, and immuno surveillance as well as epithelial to mesenchymal conversion.
The biphasic role of TGF-β in mammary tumor formation in vivo can be seen in transgenic mice that express either activated or dominant-negative forms of the TGF-β ligands and receptors. When expressed under the control of the MMTV promoter, an activated form of TβRI (MMTV-TβRIAAV) suppressed Neu-induced tumorigenesis but promoted metastasis in the tumors that formed (Siegel et al. 2003). In contrast, expression of the activated ligand (MMTV-Tgfb1223/225) did not affect tumor latency; however, tumors were more invasive and lung metastasis was more prevalent (Muraoka et al. 2003). Inducible expression of active TGF-β1 (TetOp-Tgfb1223/225) for as little as 2 weeks also increased lung metastasis in mice with polyomavirus middle-T-induced tumors, suggesting that TGF-β can rapidly promote metastatic progression (Muraoka-Cook et al. 2004). Conversly, expression of a dominant-negative form of TβRII under the control of the MMTV promoter reduced the incident of metastasis in the MMTV-neu background (Siegel et al. 2003). Conditional deletion of TβRII in mammary epithelial cells using Cre/Lox recombination (TβRIIMMTVKO) also resulted in decreased latency in polyomavirus middle-T-induced tumors (Forrester et al. 2005). Unexpectedly, increased metastasis to the lung was observed in this mouse model (Forrester et al. 2005). Several possibilities exist for the discrepancy: incomplete deletion of TβRII in tumor cells, timing for the alterations in TGF-β signaling, or cell-autonomous and non-cell-autonomous actions of TGF-β. Tobin et al. (2002) showed increased lung metastasis of MDA-231 cells in response to TGF-β irrespective of the expression of a dominant-negative receptor on the tumor cells, suggesting a role in metastasis that is paracrine-based. Nevertheless, the data suggest TGF-β is an important factor in regulating invasion and metastasis, although the exact mechanism is not clear.
Whereas it has previously been established that normal development of the mammary ductal system requires signals generated from the stroma, only recently has evidence accumulated to suggest that the stroma and microenvironment directly influence the initiation, promotion, or progression of cancer (Noel & Foidart 1998, Tlsty 2001, Tlsty & Hein 2001, Tuxhorn et al. 2001, Wiseman & Werb 2002, Erickson & Barcellos-Hoff 2003). Desmoplasia, fibroblast proliferation in conjunction with remodeling of the extracellular matrix, is often observed in human breast cancers (Noel & Foidart 1998, Erickson & Barcellos-Hoff 2003). This altered stroma is also known as the reactive stroma and has some characteristics in common with granulation tissue found during wound repair (Tuxhorn et al. 2001). It has been proposed that TGF-β expressed by cancer cells stimulates the formation of reactive stroma. TGF-β induces myo-fibroblast differentiation, extracellular matrix remodeling, and angiogenesis, properties of the reactive stroma that could promote the progression of tumors (Tuxhorn et al. 2001). Recent experimental studies in mice have implicated TGF-β signaling in the stroma as a regulator of tumor development. When epithelium-free mouse fat pads were exposed to gamma irradiation, tumor development was stimulated in a non-tumorigenic cell line, COMMA-D, suggesting that changes in the stromal environment contribute to neoplastic progression (Barcellos-Hoff & Ravani 2000). Before that, it was shown that gamma irradiation resulted in rapid remodeling of the extracellular matrix and activation of latent TGF-β in the stroma (Barcellos-Hoff 1993, Barcellos-Hoff et al. 1994). Furthermore, neutralizing antibodies directed to TGF-β suppressed matrix remodeling in response to irradiation, suggesting a role for activated TGF-β and the remodeled extracellular matrix in neoplastic progression (Ehrhart et al. 1997). Subsequently, it was shown through conditional deletion of TβRII in fibroblasts via a FSP1-cre transgene (TβRIIFSP1KO) that TGF-β signaling in the stroma modulates oncogenic potential of prostate and stomach epithelium (Bhowmick et al. 2004). Mammary cancer could not be investigated in this mouse model since the mice died at around 8 weeks of age. To access the role of TGF-β signaling to the stroma in mammary cancer, co-cultures of polyomavirus middle-T-induced mammary carcinoma cells and TβRII-positive or TβRII-deleted (TβRIIFSP1KO) fibroblasts were transplanted under the kidney capsules of nude mice (Cheng et al. 2005). TβRIIFSP1KO fibroblasts promoted tumor cell growth and invasion, which correlated with increased activation of several tyrosine kinase receptors including c-Met. Furthermore, conditioned medium from TβRIIFSP1KO fibrobasts contained high levels of Hepatocyte Growth Factor (HGF) and other growth factors and inhibitors of the growth factors blocked increased cell growth and motility in response to the conditioned media. The results suggest that TGF-β promotes mammary cancer via regulation of various growth factors in the stroma (Cheng et al. 2005). The studies together suggest that TGF-β mediates changes in the stroma that contribute to neoplastic progression.
Since TGF-β regulates invasion in mammary tumors, it has the potential to act as a therapeutic target in a clinical setting (Akhurst 2002). Clinically, tumors are generally identified at later stages of progression where the tumor-suppressive actions of TGF-β have little effect. In this regard, the concept of using soluble antagonists of TGF-β to block metastasis is appealing. It has been shown that intraperitoneal injection of a soluble chimeric protein composed of the extracellular portion of TβRII and the the Fc portion of IgG heavy chain (Fc:TβRII) blocks tumor metastasis in three different mouse models of breast cancer (Muraoka et al. 2002). Tumor cell motility and extravasation as well as matrix metalloproteinase activity were inhibited in the presence of Fc:TβRII. In addition, apoptosis in cancer cells was increased but alterations in tumor cell proliferation were not observed. Furthermore, vascular density within tumors was not affected by treatment with soluble Fc:TβRII. Transgenic mice that expressed Fc:TβRII under the control of the MMTV promoter were also resistant to metastasis compared with wild-type mice in both a tail vein metastasis assay and in crosses with MMTV-neu mice (Yang et al. 2002b). Most importantly, mice expressing Fc:TβRII did not demonstrate an increase in the formation of primary tumors and they did not demonstrate adverse side effects even though high levels of Fc:TβRII were detected in the serum. The data support the feasibility of using soluble receptors as injectable drugs in the treatment of breast cancer with few adverse effects.
Summary
Genetically engineered mice have been used to determine the role and mechanism of TGF-β signaling in normal breast physiology and pathology. DNA-regulatory elements that direct the expression of activated TGF-β ligands and receptors as well as dominant-negative forms of various signaling molecules to specific cell types and developmental stages within the mammary gland have lead to an understanding of how TGF-β regulates spatial and temporal aspects of mammary gland development. Sophisticated transplantation techniques in combination with mouse transgenic and knockout models have permitted a discussion of the importance of stromal-epithelial interaction in both normal development and tumor progression. Recent advances using Cre/Lox technology in mice have allowed conditional deletion of genes in specific tissues at specific times, providing additional information regarding TGF-β action in breast development and disease. This information is currently being used to develop strategies to combat breast cancer via the development of TGF-β antagonists (Muraoka-Cook et al. 2005). In the future, these mouse models will allow further testing of additional cancer prevention and treatment strategies.
Acknowledgments
R S is supported by NIH R01CA91974. M R C was supported by DMAD17-01-1-0201. The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
- © 2005 Society for Endocrinology