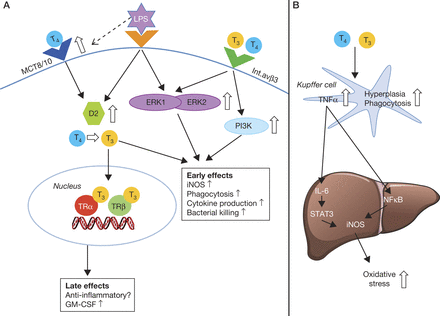
Thyroid hormone induces a pro-inflammatory response in macrophages. (A) The effects of TH in macrophages are mediated through integrin αvβ3 or through modulation of intracellular TH levels. TH can bind to integrin αvβ3 on the macrophage cell surface resulting in the activation of PI3K and ERK1/2 pathways followed by the upregulation of inducible nitric oxide synthase (iNOS) (Chen et al. 2012). Alternatively, TH can enter the cell through TH transporters MCT8 or MCT10 after which the prohormone T4 is converted to active hormone T3 by type 2 deiodinase (D2) resulting in increased phagocytosis and cytokine response. D2 is induced in lipopolysaccharide (LPS)-stimulated macrophages (Kwakkel et al. 2014). The effects of intracellular T3 are partly mediated via TRα, which is required for adequate macrophage function (Billon et al. 2014, Kwakkel et al. 2014). Low-grade inflammation found in unstimulated TRα-knockout macrophages suggests that TRα possibly attenuates the rapid pro-inflammatory response generated by increased intracellular TH levels (Billon et al. 2014). (B) Kupffer cells are the resident macrophages of the liver. TH stimulation in vivo results in Kupffer cell hyperplasia and enhanced phagocytosis (Tapia et al. 1997, Valencia et al. 2004). TH transporter and receptor expression in Kupffer cells have not yet been studied. TH also increases the production of TNFα by Kupffer cells (Valencia et al. 2004, Fernandez et al. 2005, 2007b). TNFα produced by Kupffer cells results in liver NFκB activation (Valencia et al. 2004). IL-6 production is also increased, resulting in increased STAT3 activation (Tapia et al. 2006). The activation of both these pathways results in increased iNOS activity in the liver, resulting in the production of larger amounts of reactive oxygen species and hepatic oxidative stress (Fernandez et al. 2005).











