Gene therapy of pituitary diseases
- Endocrinology, Metabolism, and Molecular Medicine, Northwestern University, Feinberg School of Medicine, Chicago, Illinois 60611, USA
- (Requests for offprints should be addressed to J L Jameson; Email: ljameson{at}northwestern.edu)
Abstract
Despite a stuttering course, gene therapy continues to provide a potential treatment avenue for many human diseases, including cancer and various inherited disorders. Gene therapy is also attractive for the treatment of local, benign disorders, such as pituitary adenomas. Advances in technology have focused on modifying existing viral vectors and developing targeted expression of therapeutic genes in an effort to achieve efficacy with minimal toxicity. Gene therapy also offers innovative strategies for treating hypopituitarism by replacing hormones such as growth hormone (GH) and vasopressin.
Introduction
The concept of gene therapy was originally introduced to treat inherited genetic disorders with the goal of replacing the defective gene with a normal one in diseased cells. This early concept has rapidly switched to a broader vision of gene therapy that uses genetic materials to alter the disease process. Currently, clinical trials are ongoing to examine the use of gene therapy in life-threatening diseases, such as malignant tumors and inherited genetic disorders.
Gene therapy for endocrine disorders, including pituitary diseases, is still at an early stage. Although a variety of pre-clinical models have been examined, there have been no attempts to use gene therapy for the treatment of pituitary diseases in humans. Pituitary cells are highly specialized and express a restricted profile of transcription factors, receptors, and hormones. For example, the anterior pituitary has five highly differentiated cell types: somatotropes (growth hormone (GH)), lactotropes (prolactin, (PRL)), corticotropes (adrenocorticotropin (ACTH)), gonadotropes (luteinizing hormone (LH) and follicle stimulating hormone (FSH)), and thyrotropes (thyroid stimulating hormone (TSH)). The posterior pituitary is a neural extension of the hypothalamus, and stores and secretes arginine vasopressin (AVP) and oxytocin. These highly-specialized features of pituitary cells afford a unique opportunity to develop targeted expression of therapeutic genes using cell type-specific approaches. Potential therapeutic targets include hormone overproduction by adenomas and hormone deficiency caused by primary pituitary or secondary hypothalamic disorders (Table 1⇓).
Causes of pituitary diseases
In this review, we describe some of the strategies for approaching pituitary gene therapy and discuss the limitations and future directions of current approaches.
Current treatment for pituitary adenomas
Current therapies for pituitary tumors include surgery, radiotherapy, and pharmacological approaches for a subset of tumor types. For microadenomas (<1 cm), transsphenoidal surgery is successful in the majority of cases (70–90%) in the hands of an experienced surgeon. However, larger macroadenomas are rarely cured by surgery alone (Lee & Jameson 1997, Melmed 1997). Medical therapies are available for selected types of pituitary tumors. In the case of prolactinomas, dopamine agonists markedly reduce hormone secretion and reduce tumor size in most patients (Schlechte 1997). However, dopamine agonists do not cure the tumors, and they recur if the medications are discontinued. Somatostatin analogues are used for the treatment of growth hormone-secreting adenomas (Melmed et al. 2002) and for TSH-secreting adenomas (Lee et al. 1994, Beck-Peccoz & Persani 2002). Finally, radiotherapy is used as an adjunctive treatment after surgery and, in rare instances, as primary treatment for pituitary tumors. Although radiotherapy is often effective, several years are usually required before it has full impact and potential complications include hypopituitarism, secondary tumors and, occasionally, neural deficits (Melmed 1997).
Gene therapy strategies for treating pituitary adenomas
Gene therapy is a seemingly reasonable strategy for the treatment of pituitary tumors. Pituitary adenomas are biologically benign, relatively localized, and they rarely metastasize. Therefore, direct administration of therapeutic vectors has a greater chance of reaching most of the tumor cells. Partial efficacy, in the absence of complete destruction of all tumor cells, might be of clinical benefit either because of reduced tumor mass or because hormone levels can be lowered. In addition, determination of hormone levels can provide a quantitative means to assess residual tumor function. As described below, the specialized nature of pituitary cells offers the opportunity to use targeted expression of therapeutic genes to the pituitary tumor mass with limited or absent expression in non-tumor cells, thereby minimizing toxicity. Key elements in the design of these approaches include the type of vector used to deliver the therapeutic gene, the type of promoter used to direct expression of the therapeutic gene and the type of therapeutic gene used to kill the tumor cells.
Viral vectors
Progress in recombinant DNA technology has made the delivery of foreign genes into normal or diseased cells a common reality. Both viral and nonviral delivery systems have been under development. Viral vectors include adenovirus, retrovirus, adeno-associated virus (AAV), lentivirus, and herpes simplex virus (HSV). Among these, adenovirus has been used most commonly for gene therapy. Adenoviruses are relatively easy to propagate and manipulate and they have a broad host range in vitro and in vivo. They have a large genome (~ 36 kb), which allows them to carry relatively large, or multiple, foreign genes. Because adenoviruses do not integrate into the host cell genome, the foreign genes delivered by adenoviral vectors are expressed in an epichromosomal manner, and therefore have low genotoxicity for host cells (Horwitz 1990, Graham & Prevec 1991, Ali et al. 1994). Because adenoviruses are expressed transiently, they are well-suited to cancer gene therapy where the goal is to destroy abnormal tissue as opposed to achieving long-term expression (as for factor IX or cystic fibrosis transmembrane conductance regulator (CFTR)). A large number of preclinical studies and clinical trials have been performed using adenoviral vectors in the treatment of malignant tumors (Kirn 2001a,b, Nemunaitis et al. 2001, Reid et al. 2001, Hamid et al. 2003, Vecil & Lang 2003). They have also been used successfully for gene transfer into normal or tumorous animal pituitary cells (Castro et al. 1997, Lee et al. 1999, 2000, Davis et al. 2001).
Despite the advantages of adenoviruses, several features limit their use in the clinical setting. These include cytotoxicity (particularly hepatoxicity), induction of immune responses, and the potential for recombination in vivo (Jolly 1994). Tissue tropism of the adenovirus is another limitation, particularly when one wishes to achieve tissue-specific effects and to minimize toxicity (Huard et al. 1995). Intravascular or inhaled administration of adenoviral vectors results in a high level of gene transfer to the liver and respiratory tract, respectively, but other targets such as the pituitary gland are not infected efficiently. Several approaches might be used in an attempt to circumvent this problem. Direct administration of adenoviral vectors into a specific organ or tissue can result in efficient infection and a high level of transgene expression (Acsadi et al. 1996, Riley et al. 1996). Injection into the carotid artery is not sufficient to convey preferential expression in the pituitary gland, presumably because of rapid dilution of the virus and entry into the general circulation (Lee et al. 2000). Although it remains possible that better vascular access could improve viral delivery, this is likely to be technically difficult and variable in its efficacy. On the other hand, stereotactic injection directly into the pituitary gland results in selective expression in the pituitary with a low level of infection in other organ such as the liver. However, with a ubiquitous promoter, such as cytomegalovirus (CMV), this approach does not preclude expression in other cell types. For this reason, additional strategies for achieving pituitary cell-specific expression have been developed (Lee et al. 2000, Davis et al. 2001).
Targeted expression strategies
Three main approaches have been used to achieve cell-specific expression in pituitary cells: (1) direct injection of viruses into the pituitary, (2) the use of pituitary hormone promoters, such as GH, proopiomelanocortin (POMC), PRL, or glycoprotein hormone α-subunit (GPHα) to direct gene expression and (3) the use of Cre-mediated recombination to activate repressed promoters in a cell-specific manner. Examples of each of these strategies are described below as pre-clinical models of pituitary tumor gene therapy. In addition to selective expression in the pituitary, it is equally important to restrict expression in tissues like the liver, which are infected with very high efficiency, to minimize toxicity from viruses designed to carry suicide genes such as thymidine kinase.
In principle, it would be desirable to ultimately modify the tissue tropism of adenoviral vectors by changing their surface charge (Schwarzenberger et al. 1996) or by performing genetic modifications (Wickham et al. 1995, 1997, Krasnykh et al. 1996). For example, the natural tropism mediated by the adenoviral fiber coat protein altered to re-target adenoviral vectors to endothelial, smooth muscle, fibroblast, and macrophage cells (Wickham et al. 1995, Wickham et al. 1997). Thus, it may be possible, using genetic modifications, to insert GH secretogogues, somatostatin, or other small molecules on the adenoviral fiber to further restrict transgene expression to the pituitary.
Therapeutic toxic genes
A variety of therapeutic genes have been developed for cancer gene therapy. These include suicide gene/prodrugs, apoptosis inducers, cytokines, and strong toxic genes (Table 2⇓). A number of suicide gene/prodrug systems were identified and are currently being used in clinical trials and preclinical studies (Morris et al. 1999). The suicide gene encodes an enzyme, usually of viral or prokaryotic origin, that is capable of converting an otherwise nontoxic prodrug into an active toxin that causes cell death. The commonly used genes that activate prodrugs include the herpes simplex virus-1 thymidine kinase (HSV-TK) gene, the Escherichia coli nitroreductase gene, the Escherichia coli cytosine deaminase gene, and Escherichia coli guanine phosphoribosyltransferase. Apoptosis-inducing genes are also likely to be good candidates for gene therapy of pituitary tumors. These include genes encoding p53, retinoblastoma (RB), dominant negative forms of growth factors, antiangiogenic factors, Fas ligand, Bax, Bcl-Xs, and caspases.
Gene therapy for pituitary tumor
Gene therapy for pituitary insufficiency
The HSV-TK gene has been used most commonly as a suicide gene/prodrug system. The cytotoxic effects of the HSV-TK gene can be activated by treatment with synthetic nucleosides such as acyclovir (ACV) or ganciclovir (GCV) (Morris et al. 1999). In the setting of cancer gene therapy, GCV is usually used because it is more effective and less toxic than ACV (Elion 1982, Biron et al. 1985). GCV is monophosphorylated by HSV-TK. An endogenous cellular kinase rapidly catalyzes subsequent phosphorylation steps (Miller & Miller 1980, 1982), leading to production of the GCV triphosphate (GCV-TP). GCV-TP is highly toxic to proliferating mammalian cells. The incorporation of the false nucleotide results in base-pair mismatches, DNA fragmentation, sister chromatid exchange, and lethal genome instability (Haynes et al. 1996). In addition, GCV inhibits cellular DNA polymerases and thereby further blocks DNA synthesis (Ilsley et al. 1995). One of the benefits of using thymidine kinase as a toxic gene is its ability to generate a bystander effect, for example, it has been shown that complete regression of tumors can be observed when only 10% of the tumor cells express thymidine kinase (Moolten 1986). This effect reflects the fact that GCV-TP can be transferred from HSV-TK positive cells to the untransduced cells through gap junctions. Cell-to-cell contact is therefore required for the bystander effect.
Recombinant viral vectors carrying suicide genes such as HSV-TK can be readily generated because they are nontoxic or minimally toxic to viral packaging cell lines, even when expressed. However, the generation of a viral vector carrying a highly toxic gene such as a diphtheria toxin is challenging, even if the toxic gene is driven by a cell-specific promoter, as nonspecific expression of even a few molecules kills the packaging cell line during generation of the recombinant viral vectors. The Cre-loxP recombinase system (see below) may afford a solution to this problem by providing a strategy to preempt the expression of the toxic gene during generation of the vector.
Preclinical models for gene therapy of pituitary tumors
Preclinical gene therapy models for the treatment of pituitary tumors have primarily used adenoviruses as vectors for gene delivery (Table 2⇑) (Riley et al. 1996, Castro et al. 1997, Lee et al. 1999, 2000, 2001a, b,c,d, Southgate et al. 2000, Windeatt et al. 2000, Davis et al. 2001, Smith-Arica et al. 2001, Southgate et al. 2001, Williams et al. 2001, Lee & Jameson 2002). In the first pituitary gene therapy model, Castro et al.(1997) demonstrated high levels of β-galactosidase gene expression in normal and tumorous (GH3 and AtT20) pituitary cells using an adenovirus carrying the β-galactosidase gene driven by ubiquitously active viral promoters. This study suggested the feasibility of using adenoviral vectors to deliver cytotoxic gene therapy to pituitary tumors. The earliest in vivo animal model used adenovirus-mediated retinoblastoma (Rb) gene therapy in spontaneous pituitary melanotrope tumors in Rb-deficient mice (Riley et al. 1996). Intratumoral RB cDNA gene delivery inhibited tumor growth and prolonged the life span of treated animals.
In subsequent studies, HSV-TK gene delivery has been most commonly used in rodent models bearing pituitary tumors implanted intradermally in nude mice or in estrogen-induced pituitary tumors in rats. The HSV-TK system has the advantage that the pro-drug activation is partially reversible by withdrawing GCV. The adenoviral vectors contain the HSV-TK gene driven by either pituitary hormone-specific promoters (GH, PRL, GPHα, and POMC) or the ubiquitously expressed CMV promoter (Lee et al. 1999, 2001c, Southgate et al. 2000, Windeatt et al. 2000). In studies using nude mice bearing pituitary tumors (GH or ACTH), GCV treatment after intratumoral injection of the adenoviral vectors containing the HSV-TK gene was highly effective, preventing further growth and destroying existing tumor cells. This apparent success may be due in part to the rapidly growing nature of pituitary tumor cell lines in nude mice and the bystander effect of HSV-TK. In contrast, a recent study found that the HSV-TK/GCV system under the control of the human prolactin promoter was not effective in suppressing prolactin levels in a rat model of lactotrope-hyperplasia (Southgate et al. 2000). This model may more closely resemble human pituitary tumors, which normally grow very slowly. However, the expression of HSV-TK by CMV promoter was effective in the same animal model (Windeatt et al. 2000), suggesting that benign pituitary adenomas might be less sensitive to the HSV-TK system unless it is expressed by a strong promoter. It should be noted, however, that high generalized expression of the TK gene product with GCV treatment may induce deleterious effects on adjacent normal pituitary cells through a bystander effect. Delivery of apoptosis-inducing or directly toxic genes may therefore be more effective for slow growing tumors.
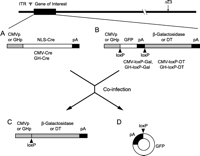
The Cre-loxP approach has two potential advantages: (1) it facilitates the production of adenoviruses carrying highly toxic genes by allowing reversible repression of promoters and (2) it provides a strategy for combinatorial activation of toxic gene expression, providing additional specificity by using separate cell-specific promoters to drive Cre expression and toxic gene expression. The Cre-loxP strategy takes advantage of the excisional deletion and recombination of a DNA sequence flanked by a pair of loxP sites, the Cre-specific recognition sequence of 34 nucleotides. A recombinant adenovirus carrying the cre recombinase gene has recently been developed and used to activate or inactivate a transgene in eukaryotic cells (Anton & Graham 1995, Sato et al. 1998a, Sato et al. 1998b, Piston et al. 1999) and animals (Stec et al. 1999). Using the Cre-loxP strategy, a two-component recombinant adenoviral system was recently developed to allow regulated activation of diphtheria toxin gene (Fig. 1⇓) (Lee & Jameson 2002). The first component contains a transcriptional blocking sequence flanked by loxP sites, interposed between the promoter and the cDNA for diphtheria toxin or a marker gene. In the second vector, Cre recombinase is driven by a cell type-specific promoter. Using co-infection of two adenoviral vectors, the marker or toxic gene was successfully activated in GH producing pituitary tumor cells in vitro and in vivo. These results indicate that Cre-mediated activation of a loxP-repressed form of the diptheria toxin (DT) gene provides a useful strategy for targeted suicide gene therapy for pituitary tumors. Diphtheria toxin-A segment (DT-A) exhibits no bystander effect, as it is unable to cross cell membranes. This feature could be advantageous in comparison to the HSV-TK/GCV system, as it might achieve selective killing of pituitary adenoma cells without harmful effects on adjacent tissues or normal pituitary cells.
Structure of recombinant adenoviral vectors and co-infection strategy. (A) Adenoviral vectors (CMV-Cre and GH-Cre) carrying the Cre recombinase gene controlled by the cytomegalovirus (CMV) promoter or the human GH promoter. (B) Adenoviral vectors (CMV-loxP-Gal, GH-loxP-Gal, CMV-loxP-DT and GH-loxP-DT) carrying the β-galactosidase gene or the DT gene. The GFP gene with an additional poly (A) signal flanked by a pair of loxP sequences was placed between the promoter and cDNA. (C, D) After co-infection of A and B, Cre recombinase excises GFP and poly (A) and recombines the promoter with the β-galactosidase or DT genes, activating their expression. ITR, inverted terminal repeat; ψ, packaging signal sequences; E1, early region 1; E3, early region 3; NLS, nuclear localized signal; DT, diphtheria toxin; pA, poly (A). (reprinted from Lee & Jameson 2002, with permission).
Prolactinomas are estrogen-dependent tumors and express estrogen receptors (ER) (Chaidarun et al. 1998, Shupnik et al. 1998). Antiestrogens including ICI 164384 and ICI 182780 have been shown to down-regulate ER, and effectively inhibit growth of pituitary GH3 cells (Newton 1995). Dominant negative forms of the ER have been suggested as an alternative method to inactivate the ER. Several dominant negative ER mutants have been reported, including truncated receptors (ER1–530 and ER1–536, missing the last 65 or 59 amino acid residues), a point mutant (L540Q), and a frame shift mutant (S554fs) (Ince et al. 1993, 1995, Chien et al. 1999, Lazennec et al. 1999). Adenovirus-mediated expression of ER1–536 or L540Q suppressed proliferation of pituitary lactotrope GH4C1 cells in vitro and in vivo (Lee et al. 2001b). Thus, it may be possible to consider expression of specific biologic modifier genes as well as more generally toxic genes. In this context, an interesting model involves delivery of the tyrosine hydroxylase (TH) gene for the treatment of prolactin-producing pituitary tumors (Freese et al. 1996). Dopamine (D2) receptor agonists are known to be effective for treating most prolactinomas. The TH gene encodes the rate-limiting enzyme in dopamine synthesis. The overexpression of the TH gene by adenovirus-mediated gene delivery suppressed prolactin levels and tumor growth in an estrogen/sirpiride-induced pituitary tumor model in rats (Williams et al. 2001).
A recent study demonstrated cell-specific expression of a marker gene in sheep after stereotactic injection of adenoviral vectors. This experiment was designed to establish a larger animal model, and extended similar approaches performed previously in rodents. In this experiment, the PRL promoter driven β-galactosidase achieved cell-specific expression with maintenance of normal endocrine function for a short period of time (Davis et al. 2001). However, there hypophysitis associated with lymphocytic infiltration and periglandular fibrosis developed during the first 7 days after adenoviral injection (Davis et al. 2002). Although a severe pituitary inflammatory reaction has not been seen in previous rodent studies, this result indicates the need for careful evaluation of the safety of gene therapy before being applied to humans. These findings also emphasize the need to explore means to reduce the immune responsiveness to adenoviruses.
Gene therapy strategies for treating pituitary insufficiency
Vector considerations for long-term hormone replacement
Long-term expression of a therapeutic gene is required for the gene therapy of pituitary insufficiency. Ideally, the gene therapy approach should also achieve regulated pituitary hormone secretion. In order to fulfill these goals, one must consider the optimal method of gene delivery, as well as the how to regulate expression. Ex vivo and in vivo strategies have been considered, the ex vivo approach involves the generation of hormone-producing cells by introducing therapeutic genes into cells from the patient and then reintroducing cells into the patient, this approach can minimize immune response. The in vivo approach requires direct administration of the vector containing therapeutic genes. Candidate vectors for in vivo delivery include: adeno-associated virus (AAV), gutted adenovirus, lentivirus, and HSV-1. AAV-2, one of the members of the Parvoviridae family, is nonpathogenic in humans and its genome integrates into human chromosomal DNA in a specific manner. Thus, the nonpathogenic nature of AAV-2, coupled with site-specific integration, provides sufficient merit to consider recombinant AAV vectors for gene therapy of pituitary insufficiency, although the packaging capacity of this vector is limited (<4.5 kb) (Carter & Samulski 2000, Rabinowitz & Samulski 2000, Buning et al. 2003).
One of the recent advances in adenoviral vector development is the use of a gutted adenoviral vector, the so called the helper-dependent (HD) system (Schiedner et al. 1998). In this vector system, the coding sequence of the adenoviral genome is completely deleted, retaining only the inverted terminal repeats (ITRs) and packaging signal sequences, which are the only essential cis-elements required for viral replication and packaging. The deleted genome is replaced with foreign DNA fragments containing the expression cassette and stuffer sequences. In order for this viral genome to be propagated and rescued in a viral particle, the proteins necessary for viral replication and packaging are needed from a helper virus. The advantage of this system is low immunogenicity, long-term gene expression, and a high packaging capacity (up to 35 kb). However, helper virus contamination is still an issue, especially for large-scale production.
Lentiviral vectors, derived from the human immunodeficiency virus (HIV), have the potential for genome integration. They can infect dividing and non-dividing cells, which is advantageous relative to existing retroviral vectors, that can only integrate into dividing cells. Currently, a gutted third generation lentiviral vector system is available (Dull et al. 1998, Zufferey et al. 1998), but its application in humans remains unproven.
HSV-1 has recently emerged as a gene therapy vector and has been exploited for gene transduction into the central nervous system. HSV-1 can persist in a latent state in neurons. Recent techniques have improved the manipulation of the large HSV genome (−150 kb) and removed the need for a helper virus. HSV-mediated marker gene expression has also been demonstrated in normal and tumorous pituitary cells in vitro and in vivo (Bolognani et al. 2001). Therefore, this vector may be a realistic option for targeted gene expression in the hypothalamus.
GH replacement approaches
Recombinant human GH (hGH) is the current treatment of choice for GH deficient patients. Recombinant hGH promotes linear growth in GH deficient children (Wyatt 2004) and it increases muscle mass and reduces adipose tissue in GH deficient adults (Hull & Harvey 2003, Merriam et al. 2004). However, the inconvenience caused by daily injections and the occasional development of anti-GH antibodies has led to consideration of a gene therapy approach (Ahangari et al. 2004). Because the targets of GH are peripheral organs, GH gene therapy does not necessarily need to be pituitary-specific. However, GH is secreted in a pulsatile fashion and has a distinct circadian rhythm that is modulated by sleep, meals, and activity. Thus, gene therapy should ideally achieve not only the restoration of normal gene expression, but also strict control of physiologic GH secretion. These are challenging goals.
The earliest approach for GH gene therapy involved the in vitro generation of GH-producing cells using myoblasts (Dhawan et al. 1991, MacColl et al. 2000), fibroblasts (Heartlein et al. 1994), or 2Y1 cells (Inazawa et al. 2001) with implantation into animals with GH deficiency. In order to minimize the host immune response, microencapsulation was employed and GH production was maintained for up to 6 months (Al-Hendy et al. 1995, 1996). In another ex-vivo approach, autologous bone marrow stromal cells were engineered to produce GH, and then transplanted back into dogs (Hurwitz et al. 1997). Because the liver synthesizes insulin like growth factor-1 (IGF-1) in response to GH, adenoviruses have been used to targeted GH expression in GH deficient lit/lit mice. Treated mice show an elevation of serum GH and IGF-1, with restoration of normal growth and weight gain (Hahn et al. 1996).
All of the approaches described above are limited by the absence of an appropriate means for regulating gene expression. Although recent attempts have included the use regulatable or tissue-specific promoters, physiologic GH secretion and production has not been fully achieved. The generation of hormone-producing cells from stem cells may provide a better solution. For example, insulin-producing cells have been produced in mouse liver after adenoviral-mediated delivery of islet-specific transcription factors (Ferber et al. 2000, Kojima et al. 2003). These insulin-producing cells appear to differentiate from progenitor cells after the introduction of β-cell lineage-specific transcription factors. These cells secrete insulin in response to blood glucose elevation. Induction of differentiation from progenitor cells using pituitary lineage specific transcription factors could provide an alternate strategy for somatotrope neogenesis, for this approach Pit-1 or PROP-1 are candidate genes. Indeed, the strategy of combining gene therapy with cellular therapy offers unique prospects for hormonal and tissue replacement (Bodine et al. 2005).
Vasopressin replacement approaches
Central diabetes insipidus is caused by the absence of synthesis or secretion of AVP from neurohypophysis. The most common cause of AVP deficiency is the destruction of the neurohypophyseal neurons and axons secondary to pituitary or hypothalamic tumors, infection, trauma, or surgical procedures. There are also rare congenital and familial forms of vasopressin deficiency. Missense and nonsense mutations in AVP neurophysin II gene cause familial neurohypophyseal diabetes insipidus (FNDI) (Ito et al. 1991, Nagasaki et al. 1995, Boson et al. 2003). While synthetic vasopressin is available and is relatively easy to administer, replacement of the AVP gene is a theoretical option for patients with central diabetes inspidus (DI). Because AVP is released by osmotic control systems, AVP gene expression should be targeted to the supraoptic nucleus (SON) or paraventricular nucleus (PVN), which receives neural input from osmoreceptors.
The AVP gene has been delivered by adenovirus to the hypothalamus of the Brattleboro rat, an AVP deficient animal model of central DI (Geddes et al. 1997), AAV (Ideno et al. 2003), or lentivirus (Bienemann et al. 2003). AVP production was documented and resulted in reduced water intake and urine volume, as well as increased urine osmolality from 4 months to 1 year. Recently, a mouse model for autosomal dominant human FNDI was established (Russell et al. 2003), providing another model for gene therapy approaches for vasopressin deficiency.
- Received 7 October 2004
- Accepted 8 November 2004
- © 2005 Society for Endocrinology