| HOME | HELP | FEEDBACK | SUBSCRIPTIONS | ARCHIVE | SEARCH | TABLE OF CONTENTS |
|
|
||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
COMMENTARY |
School of Clinical and Experimental Medicine, College of Medical and Dental Sciences, Institute of Biomedical Research, University of Birmingham, IBR Building 2nd Floor, Edgbaston, Birmingham B15 2TT, UK
(Correspondence should be addressed to K Boelaert; Email: k.boelaert{at}bham.ac.uk)
| Abstract |
|---|
| Introduction |
|---|
There are a number of well-established predictors of malignancy in thyroid nodules, including the finding of hard and fixed lesions on clinical examination, rapid growth of nodules, associated hoarseness, dysphagia or lymphadenopathy, although all of these symptoms and signs are relatively uncommon at diagnosis (Hegedus et al. 2003, Hegedus 2004). Further risk factors include young (<20 years) or old age (>70 years), male gender (Belfiore et al. 1992) and history of irradiation exposure (Ron et al. 1995). More recently, a number of studies have suggested that higher concentrations of TSH, even within the normal range, are associated with a subsequent diagnosis of thyroid cancer in patients presenting with thyroid nodules (Table 1; Boelaert et al. 2006, Jonklaas et al. 2008, Polyzos et al. 2008). Moreover, higher serum TSH levels have been found associated with advanced stages of thyroid cancer (Haymart et al. 2008a). These findings suggest that TSH may play a central role in the development and/or progression of thyroid carcinomas.
|
An alternative explanation is that patients with lower TSH concentrations already have or are progressing towards the development of one or more autonomously functioning thyroid nodules, thyroid autonomy having long been recognised as indicative of benign disease (Mann 1998, Hegedus et al. 2003). The relationship between thyroid autoimmunity and thyroid cancer has also been investigated with several studies indicating higher cancer risk in patients with Hashimoto's thyroiditis, which itself is associated with a rising TSH (Okayasu et al. 1995, Singh et al. 1999, Cipolla et al. 2005, Kurukahvecioglu et al. 2007).
In this issue of Endocrine-Related Cancer, Fiore et al. (2009) investigated 10 178 patients presenting with nodular thyroid disease and submitted to fine-needle aspiration biopsy. They report higher TSH concentrations in those with a diagnosis of PTC when compared with those with benign thyroid nodular disease (BTND), both in patients with a clinical diagnosis of multinodular goitre and in those presenting with solitary nodules. Moreover, they observed a significant age-dependent development of thyroid autoimmunity in subjects with BTND, but not in those with PTC. Serum TSH concentrations were significantly higher in PTC patients regardless of the presence of thyroid auto-antibodies and the authors conclude that thyroid autonomy protects against the risk of PTC, while thyroid autoimmunity does not play a significant role. This paper is certainly a very valuable addition to the current literature regarding this topic, although the question regarding a causal role for raised serum TSH concentrations in thyroid carcinogenesis remains unclear. This commentary aims to place the data from Fiore et al. in context, taking the available evidence into consideration, and to identify key questions that remain unanswered.
| The TSH receptor in benign and malignant thyroid tumours |
|---|
The role of serum TSH concentrations has also been extensively evaluated in thyroid nodules with a number of studies indicating a reduction in the rate of growth and prevention of new nodule formation in patients undergoing TSH suppression (Papini et al. 1998, Zelmanovitz et al. 1998, Vermiglio et al. 2003, Gharib 2004). In addition, suppressive doses of L-T4 may induce beneficial cytological changes in thyroid nodules (Vermiglio et al. 2003).
Despite these findings, there are a number of arguments to be made against a role for TSH in the development or progression of thyroid cancers. First, TSH receptor mutations in regions functionally associated with increased signal transduction do not commonly occur in thyroid carcinomas (Matsuo et al. 1993). Second, in vitro studies have shown that other growth factors such as insulin-like growth factor-I (IGF-I) have been shown to be more potent in stimulating thyroid cancer growth (Derwahl et al. 1999, Mazzaferri 2000), and TSH requires cooperation with insulin/IGF-1 to exert its proliferative effects (Kimura et al. 2001). Third, there is an inverse relationship between TSH receptor mRNA levels and cancer aggressiveness (Shi et al. 1993). Fourth, thyroid cancer is known to occur in subjects with a range of TSH concentrations including in the suppressed contralateral lobe of hyperfunctioning nodules (Satta et al. 1993). Finally, a recent genome-wide association study has indicated that serum TSH concentrations are lower in patients carrying one of two alleles associated with an increased risk of both papillary and follicular thyroid cancer (Gudmundsson et al. 2009). Taken together, these findings suggest that stimulation of the TSH receptor via increased serum TSH concentrations may play a role in the growth of benign and malignant thyroid tumours, although it is unlikely that TSH acts in isolation. It has been proposed that direct thyroid hormone-dependent cellular effects rather than TSH-mediated mechanisms are responsible for the beneficial effects in patients treated with suppressive T4 therapy (Brabant 2008).
| Serum TSH as a biochemical predictor of thyroid cancer |
|---|
These findings were subsequently confirmed by others. Haymart et al. investigated 843 patients undergoing surgery and recorded the preoperative serum TSH concentration. The likelihood of malignancy was 16% when TSH was <0.06 mIU/l, 25% for TSH between 0.40 and 1.39 mIU/l, 35% for TSH between 1.40 and 4.99 mIU/l and 52% in those with TSH of 5.0 mIU/l or greater (Haymart et al. 2008a). A further study by Polyzos et al. (2008) reported significantly increased adjusted odds ratios for the diagnosis of malignancy in those with TSH 1.5-4.0 mIU/l when compared with those with lower serum TSH concentrations at presentation. Finally, a report of a small series of 50 euthyroid patients undergoing thyroidectomy for a variety of reasons has confirmed increased risk of cancer diagnosis in subjects with serum TSH concentrations in the upper three quartiles of TSH values, compared with patients whose serum TSH was in the lower quartile. Furthermore, those who were subsequently diagnosed with thyroid cancer had lower serum concentrations of total tri-iodothyronine (T3; Jonklaas et al. 2008). Taken together, these findings suggest that serum TSH concentrations can be used as a diagnostic adjunct in the identification of high-risk patients who require further investigation and/or surgical intervention.
| Association between serum TSH and differentiated thyroid cancer stage |
|---|
Unique among all malignancies, the majority of staging systems for well-differentiated thyroid cancers includes age as one of the key prognostic factors (Dean & Hay 2000, Haymart 2009). In iodine-replete healthy adult populations, such as the cohort investigated by Haymart et al. (2008a), there is an increase in serum TSH with advancing age (Surks & Hollowell 2007, Haymart et al. 2008b). Further analysis of this cohort confirmed an association between advanced stage disease and higher serum TSH concentrations, independent of age. In addition, there was a significant correlation between higher TSH levels and extrathyroidal extension, but not with other factors associated with poor prognosis including age >45 years, tumour size >4 cm and presence of distant metastases. These findings raise the intriguing question whether higher serum TSH concentrations stimulate thyroid cancer invasion thereby facilitating extrathyroidal extension of disease.
| Serum TSH concentrations and development of thyroid autonomy |
|---|
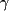 and phosphatidylinositol 3-kinase-
and phosphatidylinositol 3-kinase-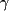 (Du Villard et al. 2000).
(Du Villard et al. 2000). Fiore et al. investigated the relationship between serum TSH and autonomous thyroid function in more detail. They found a significant age-dependent development of thyroid autonomy (TSH<0.4 µU/ml) in patients with benign thyroid disease, but this reduction of TSH with age was less evident in those with PTC. Furthermore, in patients with multinodular goitre, the frequency of thyroid autonomy was higher and the risk of PTC was lower than in those with solitary nodules (Fiore et al. 2009). Clearly, the observed decrease in serum TSH with age is at odds with data from the NHANES III survey (Hollowell et al. 2002, Aoki et al. 2007, Surks & Hollowell 2007) and one explanation may be relative iodine deficiency in the cohort investigated by Fiore et al. Others have shown higher frequency of thyroid nodularity and autonomy in older people with long-standing iodine deficiency (Fenzi et al. 1985, Aoki et al. 2007). A further explanation for the finding of decreased serum TSH in older patients is that the NHANES cohort included patients with and without nodules, whereas the current study (Fiore et al. 2009) only includes patients with thyroid nodules. The authors propose that the finding of higher serum TSH in patients with malignant thyroid disease is mainly related to a reduction of serum TSH in those with nodular goitre rather than an increase in TSH in subjects with PTC. Although the development of thyroid autonomy may slow down cancer progression, the escalating cancer risk within patients with TSH in the euthyroid and hypothyroid reference range remains unexplained.
| Autoimmune thyroid disease and thyroid cancer |
|---|
|
|
|---|
We found significantly higher rates of cancer in patients with detectable thyroid peroxidase antibodies compared with those in whom antibodies were absent, although antibody status was not an independent predictor of malignancy (Boelaert et al. 2006). In addition, Haymart et al. (2008b) found a significant association between pathological Hashimoto's thyroiditis and higher TSH levels, although thyroid antibody status was not determined in the patients investigated. In the current study by Fiore et al. (2009), the frequency of thyroid cancer was not significantly different between antibody-positive and antibody-negative patients, and higher serum TSH concentration was found in those with PTC when compared with subjects with benign disease regardless of antibody status. Taken together, the currently available data do not rule out an association between autoimmunity and thyroid cancer, but indicate that the mechanisms underlying the relationship between high serum TSH and thyroid cancer are different from those affecting the link between autoimmune thyroid disease and thyroid cancer. Clearly, further studies evaluating the role of autoimmune processes in the development and progression of thyroid cancer are required to support this.
| Conclusions |
|---|
In this issue, Fiore et al. present their findings in a large number of patients with thyroid nodules, confirming higher serum TSH concentrations in those with a diagnosis of PTC. In addition, their data indicate a lower incidence of thyroid cancer in patients with evidence of thyroid autonomy. The authors suggest that the development of thyroid autonomy, by reducing serum TSH concentrations, may represent a form of self-treatment similar to the use of suppressive doses of L-T4 in the long-term management of patients with thyroid cancer. Although plausible, this hypothesis does not explain the findings of higher rates of malignancy in patients with TSH levels in the upper half of the normal range as well as in those with subclinical and overt hypothyroidism. Further studies evaluating the role of TSH in the initiation as well as the progression of thyroid cancer are required.
| Declaration of interest |
|---|
| Funding |
|---|
| References |
|---|
Baker JR Jr 1995 The immune response to papillary thyroid cancer. Journal of Clinical Endocrinology and Metabolism 80 3419-3420.[CrossRef][Web of Science][Medline]
Balme HW 1954 Metastatic carcinoma of the thyroid successfully treated with thyroxine. Lancet 266 812-813.[Medline]
Belfiore A, La Rosa GL, La Porta GA, Giuffrida D, Milazzo G, Lupo L, Regalbuto C & Vigneri R 1992 Cancer risk in patients with cold thyroid nodules: relevance of iodine intake, sex, age, and multinodularity. American Journal of Medicine 93 363-369.[CrossRef][Web of Science][Medline]
Biondi B, Filetti S & Schlumberger M 2005 Thyroid-hormone therapy and thyroid cancer: a reassessment. Nature Clinical Practice. Endocrinology and Metabolism 1 32-40.[CrossRef]
Boelaert K, Horacek J, Holder RL, Watkinson JC, Sheppard MC & Franklyn JA 2006 Serum thyrotropin concentration as a novel predictor of malignancy in thyroid nodules investigated by fine-needle aspiration. Journal of Clinical Endocrinology and Metabolism 91 4295-4301.
Brabant G 2008 Thyrotropin suppressive therapy in thyroid carcinoma: what are the targets? Journal of Clinical Endocrinology and Metabolism 93 1167-1169.
Brewer C, Yeager N & Di CA 2007 Thyroid-stimulating hormone initiated proliferative signals converge in vivo on the mTOR kinase without activating AKT. Cancer Research 67 8002-8006.
British Thyroid Association and Royal College of Physicians 2007 Guidelines for the management of thyroid cancer. edn 2. www.british-thyroid-association.org.
Carayon P, Thomas-Morvan C, Castanas E & Tubiana M 1980 Human thyroid cancer: membrane thyrotropin binding and adenylate cyclase activity. Journal of Clinical Endocrinology and Metabolism 51 915-920.
Chang TC, Kuo SH, Liaw KY, Chang CC & Chen FW 1988 Cell kinetics, DNA content and TSH receptor-adenylate cyclase system in differentiated thyroid cancer. Clinical Endocrinology 29 477-484.[CrossRef][Medline]
Cipolla C, Sandonato L, Graceffa G, Fricano S, Torcivia A, Vieni S, Latteri S & Latteri MA 2005 Hashimoto thyroiditis coexistent with papillary thyroid carcinoma. American Surgeon 71 874-878.[Web of Science][Medline]
Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Sherman SI & Tuttle RM 2006 Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 16 1-33.[CrossRef][Web of Science][Medline]
Davies L & Welch HG 2006 Increasing incidence of thyroid cancer in the United States, 1973-2002. Journal of the American Medical Association 295 2164-2167.
Dean DS & Hay ID 2000 Prognostic indicators in differentiated thyroid carcinoma. Cancer Control 7 229-239.[Medline]
Derwahl M, Broecker M & Kraiem Z 1999 Clinical review 101: thyrotropin may not be the dominant growth factor in benign and malignant thyroid tumors. Journal of Clinical Endocrinology and Metabolism 84 829-834.
Feldt-Rasmussen U 2001 Iodine and cancer. Thyroid 11 483-486.[CrossRef][Web of Science][Medline]
Fenzi GF, Ceccarelli C, Macchia E, Monzani F, Bartalena L, Giani C, Ceccarelli P, Lippi F, Baschieri L & Pinchera A 1985 Reciprocal changes of serum thyroglobulin and TSH in residents of a moderate endemic goitre area. Clinical Endocrinology 23 115-122.[Medline]
Filetti S, Belfiore A, Amir SM, Daniels GH, Ippolito O, Vigneri R & Ingbar SH 1988 The role of thyroid-stimulating antibodies of Graves' disease in differentiated thyroid cancer. New England Journal of Medicine 318 753-759.[Web of Science][Medline]
Fiore E, Rago T, Provenzale MA, Scutari M, Ugolini C, Basolo F, Di Coscio G, Berti P, Grasso L, Elisei R, Pinchera A & Vitti P 2009 Lower levels of TSH are associated to a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: thyroid autonomy may play a protective role. Endocrine-Related Cancer 16 1251-1260.
Franceschi S 1998 Iodine intake and thyroid carcinoma - a potential risk factor. Experimental and Clinical Endocrinology and Diabetes 106 S38-S44.[CrossRef]
Gharib H 2004 Changing trends in thyroid practice: understanding nodular thyroid disease. Endocrine Practice 10 31-39.[Medline]
Goldberg RC, Lindsay S, Nichols CW Jr & Chaikoff IL 1964 Induction of neoplasms in thyroid glands of rats by subtotal thyroidectomy and by the injection of one microcurie of I-131. Cancer Research 24 35-43.
Gudmundsson J, Sulem P, Gudbjartsson DF, Jonasson JG, Sigurdsson A, Bergthorsson JT, He H, Blondal T, Geller F, Jakobsdottir M et al. 2009 Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations. Nature Genetics 41 460-464.[CrossRef][Web of Science][Medline]
Hales IB, McElduff A, Crummer P, Clifton-Bligh P, Delbridge L, Hoschl R, Poole A, Reeve TS, Wilmshurst E & Wiseman J 1992 Does Graves' disease or thyrotoxicosis affect the prognosis of thyroid cancer. Journal of Clinical Endocrinology and Metabolism 75 886-889.[Abstract]
Haymart MR 2009 Understanding the relationship between age and thyroid cancer. Oncologist 14 216-221.
Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC & Chen H 2008a Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. Journal of Clinical Endocrinology and Metabolism 93 809-814.
Haymart MR, Glinberg SL, Liu J, Sippel RS, Jaume JC & Chen H 2008b Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clinical Endocrinology[in press].
Hegedus L 2004 Clinical practice. The thyroid nodule. New England Journal of Medicine 351 1764-1771.
Hegedus L, Bonnema SJ & Bennedbaek FN 2003 Management of simple nodular goiter: current status and future perspectives. Endocrine Reviews 24 102-132.
Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA & Braverman LE 2002 Serum TSH T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). Journal of Clinical Endocrinology and Metabolism 87 489-499.
Holm LE, Blomgren H & Lowhagen T 1985 Cancer risks in patients with chronic lymphocytic thyroiditis. New England Journal of Medicine 312 601-604.[Abstract]
Hovens GC, Stokkel MP, Kievit J, Corssmit EP, Pereira AM, Romijn JA & Smit JW 2007 Associations of serum thyrotropin concentrations with recurrence and death in differentiated thyroid cancer. Journal of Clinical Endocrinology and Metabolism 92 2610-2615.
Ichikawa Y, Saito E, Abe Y, Homma M & Muraki T 1976 Presence of TSH receptor in thyroid neoplasms. Journal of Clinical Endocrinology and Metabolism 42 395-398.
Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J et al. 2006 Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 16 1229-1242.[CrossRef][Web of Science][Medline]
Jonklaas J, Nsouli-Maktabi H & Soldin SJ 2008 Endogenous thyrotropin and triiodothyronine concentrations in individuals with thyroid cancer. Thyroid 18 943-952.[CrossRef][Web of Science][Medline]
Kimura T, Van Keymeulen A, Golstein J, Fusco A, Dumont JE & Roger PP 2001 Regulation of thyroid cell proliferation by TSH and other factors: a critical evaluation of in vitro models. Endocrine Reviews 22 631-656.
Kurukahvecioglu O, Taneri F, Yuksel O, Aydin A, Tezel E & Onuk E 2007 Total thyroidectomy for the treatment of Hashimoto's thyroiditis coexisting with papillary thyroid carcinoma. Advances in Therapy 24 510-516.[CrossRef][Web of Science][Medline]
Lind P, Langsteger W, Molnar M, Gallowitsch HJ, Mikosch P & Gomez I 1998 Epidemiology of thyroid diseases in iodine sufficiency. Thyroid 8 1179-1183.[Web of Science][Medline]
Mann K 1998 Evaluation of risk in autonomously functioning thyroid nodules. Experimental and Clinical Endocrinology & Diabetes 106 S23-S26.[CrossRef]
Marqusee E, Benson CB, Frates MC, Doubilet PM, Larsen PR, Cibas ES & Mandel SJ 2000 Usefulness of ultrasonography in the management of nodular thyroid disease. Annals of Internal Medicine 133 696-700.
Matsuo K, Friedman E, Gejman PV & Fagin JA 1993 The thyrotropin receptor (TSH-R) is not an oncogene for thyroid tumors: structural studies of the TSH-R and the alpha-subunit of Gs in human thyroid neoplasms. Journal of Clinical Endocrinology and Metabolism 76 1446-1451.[Abstract]
Mazzaferri EL 1999 An overview of the management of papillary and follicular thyroid carcinoma. Thyroid 9 421-427.[Web of Science][Medline]
Mazzaferri EL 2000 Thyroid cancer and Graves' disease: the controversy ten years later. Endocrine Practice 6 221-225.[Medline]
Mazzaferri EL & Jhiang SM 1994 Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. American Journal of Medicine 97 418-428.[CrossRef][Web of Science][Medline]
Nagataki S & Nystrom E 2002 Epidemiology and primary prevention of thyroid cancer. Thyroid 12 889-896.[CrossRef][Web of Science][Medline]
Okayasu I, Fujiwara M, Hara Y, Tanaka Y & Rose NR 1995 Association of chronic lymphocytic thyroiditis and thyroid papillary carcinoma. A study of surgical cases among Japanese, and white and African Americans. Cancer 76 2312-2318.[CrossRef][Web of Science][Medline]
Papini E, Petrucci L, Guglielmi R, Panunzi C, Rinaldi R, Bacci V, Crescenzi A, Nardi F, Fabbrini R & Pacella CM 1998 Long-term changes in nodular goiter: a 5-year prospective randomized trial of levothyroxine suppressive therapy for benign cold thyroid nodules. Journal of Clinical Endocrinology and Metabolism 83 780-783.
Pellegriti G, Belfiore A, Giuffrida D, Lupo L & Vigneri R 1998 Outcome of differentiated thyroid cancer in Graves' patients. Journal of Clinical Endocrinology and Metabolism 83 2805-2809.
Polyzos SA, Kita M, Efstathiadou Z, Poulakos P, Slavakis A, Sofianou D, Flaris N, Leontsini M, Kourtis A & Avramidis A 2008 Serum thyrotropin concentration as a biochemical predictor of thyroid malignancy in patients presenting with thyroid nodules. Journal of Cancer Research and Clinical Oncology 134 953-960.[CrossRef][Web of Science][Medline]
Pujol P, Daures JP, Nsakala N, Baldet L, Bringer J & Jaffiol C 1996 Degree of thyrotropin suppression as a prognostic determinant in differentiated thyroid cancer. Journal of Clinical Endocrinology and Metabolism 81 4318-4323.[Abstract]
Reiners C, Wegscheider K, Schicha H, Theissen P, Vaupel R, Wrbitzky R & Schumm-Draeger PM 2004 Prevalence of thyroid disorders in the working population of Germany: ultrasonography screening in 96,278 unselected employees. Thyroid 14 926-932.[CrossRef][Web of Science][Medline]
Ron E, Lubin JH, Shore RE, Mabuchi K, Modan B, Pottern LM, Schneider AB, Tucker MA & Boice JD Jr 1995 Thyroid cancer after exposure to external radiation: a pooled analysis of seven studies. Radiation Research 141 259-277.[Web of Science][Medline]
Ross DS 2006 Predicting thyroid malignancy. Journal of Clinical Endocrinology and Metabolism 91 4253-4255.
Sanders LE & Cady B 1998 Differentiated thyroid cancer: reexamination of risk groups and outcome of treatment. Archives of Surgery 133 419-425.
Satta MA, De RG, Testa A, Maussier ML, Valenza V, Rabitti C, Saletnich I, D'Ugo D & Picciocchi A 1993 Thyroid cancer in suppressed contralateral lobe of patients with hot thyroid nodule. European Journal of Cancer 29A 1190-1192.[CrossRef]
Shi Y, Zou M & Farid NR 1993 Expression of thyrotrophin receptor gene in thyroid carcinoma is associated with a good prognosis. Clinical Endocrinology 39 269-274.[Medline]
Simpson WJ, Panzarella T, Carruthers JS, Gospodarowicz MK & Sutcliffe SB 1988 Papillary and follicular thyroid cancer: impact of treatment in 1578 patients. International Journal of Radiation Oncology, Biology, Physics 14 1063-1075.[Web of Science][Medline]
Singh B, Shaha AR, Trivedi H, Carew JF, Poluri A & Shah JP 1999 Coexistent Hashimoto's thyroiditis with papillary thyroid carcinoma: impact on presentation, management, and outcome. Surgery 126 1070-1076.[CrossRef][Web of Science][Medline]
Surks MI & Hollowell JG 2007 Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. Journal of Clinical Endocrinology and Metabolism 92 4575-4582.
Tunbridge WM, Evered DC, Hall R, Appleton D, Brewis M, Clark F, Evans JG, Young E, Bird T & Smith PA 1977 The spectrum of thyroid disease in a community: the Whickham survey. Clinical Endocrinology 7 481-493.[Medline]
Vander JB, Gaston EA & Dawber TR 1968 The significance of nontoxic thyroid nodules. Final report of a 15-year study of the incidence of thyroid malignancy. Annals of Internal Medicine 69 537-540.
Vermiglio F, Lo P, Violi MA, Moleti M, Castagna MG, Finocchiaro MD, Mattina F, Mandolfino M, Zimbaro G & Trimarchi F 2003 Changes in both size and cytological features of thyroid nodule after levothyroxine treatment. Clinical Endocrinology 59 347-353.[CrossRef][Medline]
Du Villard JA, Wicker R, Crespo P, Russo D, Filetti S, Gutkind JS, Sarasin A & Suarez HG 2000 Role of the cAMP and MAPK pathways in the transformation of mouse 3T3 fibroblasts by a TSHR gene constitutively activated by point mutation. Oncogene 19 4896-4905.[CrossRef][Web of Science][Medline]
Walker RP & Paloyan E 1990 The relationship between Hashimoto's thyroiditis, thyroid neoplasia, and primary hyperparathyroidism. Otolaryngologic Clinics of North America 23 291-302.[Web of Science][Medline]
Yano Y, Shibuya H, Kitagawa W, Nagahama M, Sugino K, Ito K & Ito K 2007 Recent outcome of Graves' disease patients with papillary thyroid cancer. European Journal of Endocrinology 157 325-329.
Yeager N, Klein-Szanto A, Kimura S & Di CA 2007 Pten loss in the mouse thyroid causes goiter and follicular adenomas: insights into thyroid function and Cowden disease pathogenesis. Cancer Research 67 959-966.
Zelmanovitz F, Genro S & Gross JL 1998 Suppressive therapy with levothyroxine for solitary thyroid nodules: a double-blind controlled clinical study and cumulative meta-analyses. Journal of Clinical Endocrinology and Metabolism 83 3881-3885.
This article has been cited by other articles:
 |
H. Raef, R. Al-Rijjal, S. Al-shehri, M. Zou, H. Al-Mana, E. Y. Baitei, R. S. Parhar, F. A. Al-Mohanna, and Y. Shi Biallelic p.R2223H Mutation in the Thyroglobulin Gene Causes Thyroglobulin Retention and Severe Hypothyroidism with Subsequent Development of Thyroid Carcinoma J. Clin. Endocrinol. Metab., March 1, 2010; 95(3): 1000 - 1006. [Abstract] [Full Text] [PDF] |
||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HOME | HELP | FEEDBACK | SUBSCRIPTIONS | ARCHIVE | SEARCH | TABLE OF CONTENTS |